Abstract
Seventy-six patients with acute promyelocytic leukemia (APL) in first complete remission after induction and consolidation by daunorubicin and cytosine arabinoside received oral arsenic trioxide (As2O3)-based maintenance. Three regimens were used: oral As2O3 (10 mg/day, regimen A, n = 20), oral As2O3 plus all-trans retinoic acid (ATRA, 45 mg/m2 per day, regimen AA, n = 19), and oral As2O3 plus ATRA plus ascorbic acid (1000 mg/day, regimen AAA, n = 37), each given for 2 weeks every 2 months for 2 years. Patients receiving A, AA, and AAA maintenance did not differ significantly in clinicopathologic features and risk factors. Headache, dyspepsia, reversible liver function derangement, and herpes zoster reactivation were adverse effects observed during maintenance. QTc prolongation and arrhythmias were not encountered. At a median follow-up of 24 months (range, 1-115 months), there were 8 relapses. The 3-year leukemia-free-survival, event-free-survival, and overall-survival were 87.7%, 83.7%, and 90.6%, respectively. Adverse prognostic factors included male gender for leukemia-free-survival, and unrelated cancers for overall survival. Age, presentation WBC count and platelet count, and the type of oral As2O3 maintenance regimens had no impact on survivals. Prolonged oral As2O3 maintenance was feasible and safe and resulted in favorable outcomes when used with a simple induction and consolidation regimen compared with other protocols composed of multiple chemotherapeutic agents.
Introduction
Acute promyelocytic leukemia (APL) is a potentially curable leukemia. With the use of all-trans retinoic acid (ATRA) and chemotherapy, and the careful management of bleeding complications and the APL differentiation syndrome,1,2 complete remission (CR) rates of > 90% can be achieved. Therefore, prevention of relapse has become an important management issue for APL patients in CR.
Relapse in APL has been shown to be related to presentation clinical features, including age, high WBC (> 10 × 109/L), low platelet count (< 40 × 109/L),3 and persistence of PML-RARA fusion gene after consolidation therapy.4 The relapse rate of these high-risk persons might be as high as 30% to 40%.2,3
To improve the outcome of APL, risk-adapted strategies have been used in several treatment regimens, including the PETHEMA LPA99,5 European APL group APL2000,6 PETHEMA and HOVON LPA2005,7 and GIMEMA AIDA-20008 protocols. Standard-risk patients received at least 2 or 3 courses of consolidation composed of an anthracycline (idarubicin or daunorubicin), and in some protocols a topoisomerase II inhibitor mitoxantrone, and cytosine arabinoside (Ara-C) at 1 to 1.5 g/m2. For high-risk patients, more drugs and/or drugs at higher doses were administered, including Ara-C during induction7 ; increased doses of idarubicin,5 Ara-C,6,7 and mitoxantrone7 ; and additional use of etoposide and thioguanine8 during consolidation. With a risk-adapted approach, the relapse rate even for high-risk persons has decreased to 10.7% to 24%.5-8
Arsenic trioxide (As2O3) has been shown to be highly effective for relapsed APL, inducing a remission in up to 90% of patients.9 Because of its high efficacy, the role of As2O3 has been examined in frontline treatment of APL. Combined ATRA and As2O3 induced CR with significantly better reduction of PML-RARA.10 The addition of gemtuzumab ozogamicin to ATRA plus As2O3 also showed high efficacy in newly diagnosed APL.11 Furthermore, As2O3 used as a single agent without additional chemotherapy resulted in high remission rates and reasonable long-term outcomes.12,13
Based on favorable results of the frontline use of As2O3, recent studies have examined the impact of including As2O3 in consolidation regimens. The addition of a 30-day course of As2O3 to a standard daunorubicin/Ara-C consolidation regimen resulted in a 3-year disease-free survival (DFS) of nearly 90% and enabled a smaller dose of daunorucibin to be administered.14 In the North America Intergroup Study C9710, the addition of two 25-day courses of As2O3 improved the 3-year event-free-survival (EFS) from 63% in the control group to 80% in the As2O3 group.15 These results showed that the early use of As2O3 might be another approach for improving the outcome of APL patients.
In all previous studies, the intravenous formulation of As2O3 has been used. We have developed an oral formulation of As2O3 for the treatment of relapsed APL.16 Oral As2O3 is well absorbed and achieved a bioavailability, as measured by area under the curve pharmacokinetically, of up to 95% of an equivalent dose of intravenous As2O3.17 Because slow oral absorption results in lower peak plasma arsenic levels compared with intravenous As2O3, oral As2O3 is associated with minimal prolongation of QT interval,18,19 making the medication suitable for treatment at home without the need of daily hospital visit (as for intravenous As2O3) and monitoring for QT prolongation or cardiac arrhythmias.
Because of the high efficacy of oral As2O3 in relapsed APL, we surmised that the use of oral As2O3 in APL patients in CR1 might decrease the incidence of relapse and therefore improve treatment outcome. In this study, we report our experience of the use of oral As2O3-based regimens in the maintenance treatment of a cohort of APL patients in CR1.
Methods
Patients
APL was diagnosed by standard morphologic, cytogenetic, and molecular criteria as stipulated by the World Health Organization classification criteria. Patients 65 years of age or younger were treated with ATRA (45 mg/m2 per day, administered from day 1 until CR) and a standard 3:7 protocol (3 days of daunorubicin at 50 mg/m2 per day, 7 days of cytosine arabinoside at 100 mg/m2 per day). This was followed by 2 monthly courses of 2:5 consolidation (2 days of daunorubicin at 50 mg/m2 per day, 5 days of cytosine arabinoside at 100 mg/m2 per day) without ATRA. Patients older than 65 years of age had the 2 courses of 2:5 consolidation omitted. Furthermore, patients older than 70 years received ATRA (45 mg/m2 per day) and oral As2O3 (10 mg/day) until CR, and then maintenance without consolidation. From 2001 to 2010, all consecutively diagnosed APL patients reaching CR1 after induction and consolidation (if given) were registered and received maintenance treatment based on oral As2O3. There were otherwise no inclusion or exclusion criteria. The use of oral As2O3 was approved by the Institutional Review Board at Queen Mary Hospital, Hong Kong.
Maintenance regimens
During the 10-year study period, 3 maintenance regimens had evolved, each entailing drug treatment for 2 weeks every 2 months for 2 years. These regimens were composed of oral As2O3 (10 mg/day, regimen A, used during 2001-2004), oral As2O3 (10 mg/day) plus ATRA (45 mg/m2 per day, regimen AA, used during 2005-2007), and oral As2O3 (10 mg/day) plus ATRA (45 mg/m2 per day) plus ascorbic acid (1 g/d, regimen AAA, used from 2008 onwards).
Dose reduction
Because As2O3 is renal-excreted, patients older than 70 years or with a serum creatinine concentration exceeding the upper reference range (male, 109μM; female, 82μM) would receive a 50% reduction in the dose of oral As2O3. Headache was the commonest side effect of ATRA, and patients with headache not relieved by simple analgesia would have the dose of ATRA reduced by 50%.
Molecular monitoring
PCR for the PML-RARA fusion transcript was performed on the peripheral blood obtained at each follow-up as described.18 Molecular relapse was defined as PML-RARA positivity that could be verified in a subsequent consecutive sample taken in 2 weeks. Clinical frank relapses were proven morphologically and molecularly.
Statistical analysis
All data were censored on December 31, 2010. Data were analyzed on an intention-to-treat model. Overall survival (OS) was defined as the time from study entry to death. EFS was defined as the time from CR1 to relapse (clinical or molecular) or death. Leukemia-free-survival (LFS) was defined as the time from the end of induction to relapse (clinical or molecular) or death because of leukemia. Deaths from nonleukemic causes were not considered as events in the analysis of LFS because patients who were very old or had active or terminal malignancies were included, which would be strong competing risks for death. Analysis of survivals (OS, EFS, and LFS) was conducted by the Kaplan-Meier method. Differences in OS, EFS, and LFS were tested by log-rank analysis with the use of Cox proportional hazards models. Fisher exact test was used for analysis of categorical variables. Two-tailed P values of < .05 were taken as being significant. All tests were performed with the SPSS Version 15.0 software package.
Results
Patients
Seventy-six patients in CR1 received oral As2O3 maintenance. Their clinicopathologic characteristics are shown in Table 1. As a cohort, the median age was 44 years (16-83 years). At presentation, the median hemoglobin was 8.0 g/dL (range, 3.3-14.6 g/dL), the median leukocyte count 2.5 × 109/L (range, 0.3-121 × 109/L), and the median platelet count 22 × 109/L (range, 3-173 × 109/L). Six patients had a previous malignancy treated with chemotherapy, so that their APL was considered therapy-related. Of the 70 patients with de novo APL, 4 had a previous malignancy (carcinoma of liver, breast, uterus, and colon) that was treated surgically only. At presentation, 4 elderly patients (74-83 years old) received ATRA plus As2O3 induction without chemotherapy for a median of 31 days (range, 25-42 days), and all of them achieved CR. Consolidation was omitted in 6 patients (65-73 years old) who also had other comorbidities (including heart failure, refractory breast cancer, and severe bronchiectasis). Three patients received only 1 course of consolidation because of toxicity or refusal for further chemotherapy. During induction, leukocytosis (> 10 × 109/L) occurred in 35 patients, with 16 cases requiring treatment with dexamethasone (4-8 mg intravenously every 8 hours) for the APL differentiation syndrome.
Clinicopathologic characteristics of 76 patients with APL receiving different As2O3-containing maintenance regimens
| Parameters . | Value . |
|---|---|
| As2O3 maintenance (regimen A, N = 20) | |
| Male/female | 12:8 |
| Median age, y (range) | 43 (26-73) |
| Median hemoglobin, g/dL (range) | 8.1 (5.4-12.3) |
| Median WBC count, × 109/L (range) | 1.8 (0.3-30) |
| Median platelet count, × 109/L (range) | 16 (6-162) |
| WBC count, > 10 × 109/L | 2 |
| Platelet count, < 40 × 109/L | 14 |
| Previous induction with chemotherapy + ATRA | 20 |
| Leukocytosis > 10 × 109/L during treatment | 8 |
| Median peak WBC count during induction, × 109/L (range) | 6.5 (1.2-90) |
| APL differentiation syndrome | 5 |
| As2O3 + ATRA maintenance (regimen AA, N = 19) | |
| Male/female | 8:11 |
| Median age, y (range) | 54 (16-73) |
| Median hemoglobin, g/dL (range) | 8 (4.8-13.4) |
| Median WBC count, × 109/L (range) | 4.7 (0.5-62) |
| Platelet count, × 109/L, median (range) | 23 (3-78) |
| WBC count, > 10 × 109/L | 5 |
| Platelet count, < 40 × 109/L | 14 |
| Previous induction with chemotherapy + ATRA | 19 |
| Leukocytosis > 10 × 109/L during treatment | 11 |
| Median peak WBC count during induction, × 109/L (range) | 21 (0.8-70) |
| APL differentiation syndrome | 6 |
| As2O3 + ATRA + ascorbic acid maintenance (regimen AAA, N = 37) | |
| Male/female | 15:22 |
| Median age, y (range) | 48 (23-83) |
| Median hemoglobin, g/dL (range) | 7.8 (3.3-14.6) |
| Median WBC count, × 109/L (range) | 19 (0.3-121) |
| Median platelet count, × 109/L (range) | 26 (8-112) |
| WBC count, > 10 × 109/L | 3 |
| Platelet count, < 40 × 109/L | 23 |
| Previous induction with chemotherapy + ATRA | 33 |
| Previous induction with As2O3 + ATRA | 4 |
| Leukocytosis > 10 × 109/L during treatment | 16 |
| Median peak WBC count during induction, × 109/L (range) | 9.2 (0.6-123) |
| APL differentiation syndrome | 5 |
| Parameters . | Value . |
|---|---|
| As2O3 maintenance (regimen A, N = 20) | |
| Male/female | 12:8 |
| Median age, y (range) | 43 (26-73) |
| Median hemoglobin, g/dL (range) | 8.1 (5.4-12.3) |
| Median WBC count, × 109/L (range) | 1.8 (0.3-30) |
| Median platelet count, × 109/L (range) | 16 (6-162) |
| WBC count, > 10 × 109/L | 2 |
| Platelet count, < 40 × 109/L | 14 |
| Previous induction with chemotherapy + ATRA | 20 |
| Leukocytosis > 10 × 109/L during treatment | 8 |
| Median peak WBC count during induction, × 109/L (range) | 6.5 (1.2-90) |
| APL differentiation syndrome | 5 |
| As2O3 + ATRA maintenance (regimen AA, N = 19) | |
| Male/female | 8:11 |
| Median age, y (range) | 54 (16-73) |
| Median hemoglobin, g/dL (range) | 8 (4.8-13.4) |
| Median WBC count, × 109/L (range) | 4.7 (0.5-62) |
| Platelet count, × 109/L, median (range) | 23 (3-78) |
| WBC count, > 10 × 109/L | 5 |
| Platelet count, < 40 × 109/L | 14 |
| Previous induction with chemotherapy + ATRA | 19 |
| Leukocytosis > 10 × 109/L during treatment | 11 |
| Median peak WBC count during induction, × 109/L (range) | 21 (0.8-70) |
| APL differentiation syndrome | 6 |
| As2O3 + ATRA + ascorbic acid maintenance (regimen AAA, N = 37) | |
| Male/female | 15:22 |
| Median age, y (range) | 48 (23-83) |
| Median hemoglobin, g/dL (range) | 7.8 (3.3-14.6) |
| Median WBC count, × 109/L (range) | 19 (0.3-121) |
| Median platelet count, × 109/L (range) | 26 (8-112) |
| WBC count, > 10 × 109/L | 3 |
| Platelet count, < 40 × 109/L | 23 |
| Previous induction with chemotherapy + ATRA | 33 |
| Previous induction with As2O3 + ATRA | 4 |
| Leukocytosis > 10 × 109/L during treatment | 16 |
| Median peak WBC count during induction, × 109/L (range) | 9.2 (0.6-123) |
| APL differentiation syndrome | 5 |
Maintenance regimens
Maintenance treatment was started at a median of 25 days (15-42 days) after the last chemotherapy. The clinicopathologic features of patients receiving A, AA, and AAA maintenance regimens were presented in Table 1. There were no significant differences in the 3 groups with respect to demographics, clinicopathologic features, and previous treatment before relapse (Table 2). There were no patient drop-offs from the maintenance therapy.
Clinicopathologic features and adverse effects of different oral As2O3 maintenance regimens in 76 patients
| . | Regimen A (N = 20) . | Regimen AA (N = 19) . | Regimen AAA (N = 37) . | P . |
|---|---|---|---|---|
| Clinicopathologic features | ||||
| Male | 12 | 8 | 15 | NS (.34) |
| Age > 40 y | 13 | 13 | 24 | NS (.96) |
| Presentation WBC count > 10 × 109/L | 2 | 5 | 3 | NS (.14) |
| Presentation platelet count < 40 × 109/L | 14 | 14 | 23 | NS (.65) |
| APL differentiation syndrome | 5 | 6 | 5 | NS (.26) |
| Peak WBC count > 10 × 109/L | 8 | 11 | 16 | NS (.48) |
| Second solid tumor (before/after) maintenance | 3 | 3 | 8 | NS (.78) |
| Adverse effects | ||||
| Headache | 3 | 11 | 8 | .005 |
| Liver function derangement | 6 | 3 | 8 | NS (.56) |
| Herpes zoster reactivation | 2 | 5 | 3 | NS (.14) |
| Rash | 2 | 3 | 3 | NS (.67) |
| Dyspepsia | 3 | 4 | 11 | NS (.43) |
| . | Regimen A (N = 20) . | Regimen AA (N = 19) . | Regimen AAA (N = 37) . | P . |
|---|---|---|---|---|
| Clinicopathologic features | ||||
| Male | 12 | 8 | 15 | NS (.34) |
| Age > 40 y | 13 | 13 | 24 | NS (.96) |
| Presentation WBC count > 10 × 109/L | 2 | 5 | 3 | NS (.14) |
| Presentation platelet count < 40 × 109/L | 14 | 14 | 23 | NS (.65) |
| APL differentiation syndrome | 5 | 6 | 5 | NS (.26) |
| Peak WBC count > 10 × 109/L | 8 | 11 | 16 | NS (.48) |
| Second solid tumor (before/after) maintenance | 3 | 3 | 8 | NS (.78) |
| Adverse effects | ||||
| Headache | 3 | 11 | 8 | .005 |
| Liver function derangement | 6 | 3 | 8 | NS (.56) |
| Herpes zoster reactivation | 2 | 5 | 3 | NS (.14) |
| Rash | 2 | 3 | 3 | NS (.67) |
| Dyspepsia | 3 | 4 | 11 | NS (.43) |
Regimen A indicates As2O3; Regimen AA, ATRA + As2O3; and Regimen AAA, ATRA + As2O3 + ascorbic acid.
Adverse events observed during maintenance
Electrocardiograms, electrolytes, and liver and renal function tests were checked on every outpatient visit. Adverse effects included headache (n = 22, 29%), dyspepsia (n = 18, 24%), reversible liver function derangement (n = 17, 22%), herpes zoster reactivation20 (n = 10, 13%), rash (n = 8, 11%), and menorrhagia (n = 3, 4%) (Table 2). None of the adverse effects was serious enough to warrant treatment cessation. Clinical arrhythmias were not observed, and QTc prolongation was not detectable by electrocardiogram monitoring in any patients. Headache was significantly less frequent for regimen A compared with regimens AA and AAA (P = .005). Five solid tumors were diagnosed during the study period (colon cancer, n = 2; salivary gland cancer, n = 1; breast carcinoma, n = 1, nasopharyngeal carcinoma n = 1). Only the patient with breast carcinoma survived.
Molecular monitoring
On referral for As2O3 maintenance, none of the cases was positive for PML-RARA by PCR. Subsequent monitoring detected peripheral blood PML-RARA positivity in 21 patients (during maintenance, n = 11; off maintenance, n = 10), at a median of 19 months (range, 2-68 months) of follow-up. For the 11 patients who tested positive for PML-RARA during maintenance, a repeat sample in 2 weeks was negative. Oral As2O3 maintenance was continued uninterrupted. Ten patients did not have any further tests positive for PML-RARA and had remained in remission. However, 1 patient (unique progression number 5 [UPN 5]) had subsequent tests again positive for PML-RARA and ultimately relapsed systemically (Table 3). For the 10 patients who tested PML-RARA positive while off maintenance, 8 patients were negative for PML-RARA on a subsequent sample. PML-RARA had remained negative for a median of 11 months (4-14 months). One patient (UPN 24) continued to be PML-RARA positive and had a subsequent CNS and marrow relapse (Table 3). The last patient (UPN 22) was also persistently PML-RARA positive. She was treated as a case of molecular relapse and put back on AAA treatment, resulting in reversion to PML-RARA negativity (Table 3). In 4 other patients (UPN 10, UPN 19, UPN 37, and UPN 56), relapses occurred without a preceding PML-RARA positivity. As patients were followed up every 2 months, it could be possible that the tempo of relapse precluded detection by PCR.
Relapses or death of 13 APL patients in CR1 receiving maintenance treatment with oral As2O3
| UPN . | Sex . | Age, y . | Presentation . | Peak WBC during induction treatment . | APL differentiation syndrome . | Maintenance . | First site of relapse . | Event (time) . | Treatment and outcome . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin, g/dL . | WBC, × 109/L . | Platelets, × 109/L . | |||||||||
| Relapse | |||||||||||
| 5 | M | 44 | 7.0 | 5.1 | 26 | 90.0 | Yes | A | Marrow | Relapse (20 mo) | CR2 with AAA, 3 subsequent CNS relapses at 36 mo, 68 mo, 89 mo; re-treated with AAA + RT and IT chemotherapy to CR3, died of systemic relapse at 100 mo |
| 10 | M | 42 | 11.0 | 1.2 | 162 | 4.0 | No | A | Marrow | Relapse (11 mo) | CR2 with AAA, died of pulmonary embolism at 16 mo |
| 19 | M | 44 | 9.9 | 1.7 | 7 | 22.3 | No | A | Marrow | Relapse (12 mo) | CR2 with AAA, third systemic and CNS relapse at 30 mo, also developed salivary gland carcinoma, died of APL at 37 mo |
| 24 | M | 58 | 8.6 | 19.8 | 78 | 33.2 | No | AA | Marrow | Relapse (27 mo)* | CR2 with AAA, CNS relapse at 52 mo, CR3 with AAA + IT + RT + chemotherapy, in CR3 at 66+ mo |
| 37 | M | 49 | 8.0 | 2.4 | 13 | 61.1 | Yes | AA | CNS and marrow | Relapse (27 mo)* | CR2 with AAA + RT + chemotherapy, 43+ mo |
| 43 | M | 42 | 7.9 | 121.0 | 19 | 123.0 | Yes | AAA | CNS | Relapse (0 mo)† | CR2 with IT + chemotherapy + RT, 34+ mo |
| 56 | M | 39 | 3.3 | 1.0 | 8 | 1.1 | No | AAA | Marrow | Relapse (11 mo) ‡ | CR2 with AAA, 20+ mo |
| 22 | F | 40 | 9.5 | 0.7 | 51 | 6.7 | No | AA | Molecular | Mo relapse (55 mo) | CR2 with AAA, 15+ mo |
| Death | |||||||||||
| 1 | M | 72 | 9.7 | 1.0 | 123 | 2.0 | No | A | Death (89 mo) | Died of colon cancer in CR1, also had history of mantle cell lymphoma | |
| 2 | F | 73 | 5.4 | 0.3 | 62 | 4.0 | No | A | Death (62 mo) | Died of a stroke, in CR1 | |
| 12 | F | 44 | 8.0 | 1.2 | 82 | 1.7 | Yes | A | Death (33 mo) | Died of nasopharyngeal cancer, in CR1 | |
| 41 | F | 47 | 6.4 | 6.8 | 39 | 35.0 | No | AAA | Death (18 mo) | Died of colon cancer, in CR1 | |
| 69 | F | 53 | 8.8 | 0.8 | 14 | 9.2 | No | AAA | Death (4 mo) | Died of disseminated breast cancer predating the diagnosis of APL, in CR1 | |
| UPN . | Sex . | Age, y . | Presentation . | Peak WBC during induction treatment . | APL differentiation syndrome . | Maintenance . | First site of relapse . | Event (time) . | Treatment and outcome . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin, g/dL . | WBC, × 109/L . | Platelets, × 109/L . | |||||||||
| Relapse | |||||||||||
| 5 | M | 44 | 7.0 | 5.1 | 26 | 90.0 | Yes | A | Marrow | Relapse (20 mo) | CR2 with AAA, 3 subsequent CNS relapses at 36 mo, 68 mo, 89 mo; re-treated with AAA + RT and IT chemotherapy to CR3, died of systemic relapse at 100 mo |
| 10 | M | 42 | 11.0 | 1.2 | 162 | 4.0 | No | A | Marrow | Relapse (11 mo) | CR2 with AAA, died of pulmonary embolism at 16 mo |
| 19 | M | 44 | 9.9 | 1.7 | 7 | 22.3 | No | A | Marrow | Relapse (12 mo) | CR2 with AAA, third systemic and CNS relapse at 30 mo, also developed salivary gland carcinoma, died of APL at 37 mo |
| 24 | M | 58 | 8.6 | 19.8 | 78 | 33.2 | No | AA | Marrow | Relapse (27 mo)* | CR2 with AAA, CNS relapse at 52 mo, CR3 with AAA + IT + RT + chemotherapy, in CR3 at 66+ mo |
| 37 | M | 49 | 8.0 | 2.4 | 13 | 61.1 | Yes | AA | CNS and marrow | Relapse (27 mo)* | CR2 with AAA + RT + chemotherapy, 43+ mo |
| 43 | M | 42 | 7.9 | 121.0 | 19 | 123.0 | Yes | AAA | CNS | Relapse (0 mo)† | CR2 with IT + chemotherapy + RT, 34+ mo |
| 56 | M | 39 | 3.3 | 1.0 | 8 | 1.1 | No | AAA | Marrow | Relapse (11 mo) ‡ | CR2 with AAA, 20+ mo |
| 22 | F | 40 | 9.5 | 0.7 | 51 | 6.7 | No | AA | Molecular | Mo relapse (55 mo) | CR2 with AAA, 15+ mo |
| Death | |||||||||||
| 1 | M | 72 | 9.7 | 1.0 | 123 | 2.0 | No | A | Death (89 mo) | Died of colon cancer in CR1, also had history of mantle cell lymphoma | |
| 2 | F | 73 | 5.4 | 0.3 | 62 | 4.0 | No | A | Death (62 mo) | Died of a stroke, in CR1 | |
| 12 | F | 44 | 8.0 | 1.2 | 82 | 1.7 | Yes | A | Death (33 mo) | Died of nasopharyngeal cancer, in CR1 | |
| 41 | F | 47 | 6.4 | 6.8 | 39 | 35.0 | No | AAA | Death (18 mo) | Died of colon cancer, in CR1 | |
| 69 | F | 53 | 8.8 | 0.8 | 14 | 9.2 | No | AAA | Death (4 mo) | Died of disseminated breast cancer predating the diagnosis of APL, in CR1 | |
Regimen A indicates As2O3; Regimen AA, ATRA + As2O3; Regimen AAA, ATRA + As2O3 + ascorbic acid; CR1, first complete remission; CR2, second complete remission; IT, intrathecal chemotherapy; RT, cranial radiotherapy; M, male; F, female; and mo, molecular.
Relapse after cessation of 24 months of maintenance.
Relapse discovered on referral.
Confessed to poor compliance with maintenance drugs.
Relapses
At a median follow-up of 24 months (range, 1-115 months), there were 8 relapses (bone marrow, n = 5; CNS, n = 1; marrow + CNS, n = 1; molecular, n = 1; Table 3). Interestingly, all frank relapses occurred in male patients, whereas the molecular relapse occurred in a female patient. One patient on referral was already found to have isolated CNS relapse before maintenance treatment could be administered. The median time to relapse was 18 months (range, 0-55 months) from CR1. Four patients relapsed while on maintenance. Three of them were taking oral As2O3 only. The other patient was on AAA maintenance but admitted to poor drug compliance, so that the actual amount of drug taken was unknown. Three other patients relapsed after finishing 2 years of AA maintenance, including the case with molecular relapse.
Treatment and outcome of relapses
Relapsed patients were reinduced with the AAA regimen, supplemented by intrathecal chemotherapy and cranial irradiation in patients with CNS disease. All 8 patients achieved CR2, with no detectable PML-RARA. Three patients developed a second relapse (R2), presenting as CNS disease in 2 patients and marrow and CNS disease in 1 patient. Even in these advanced relapses, the AAA regimen still retained its efficacy and 2 of the 3 cases achieved a CR3.
Risks for relapses while on oral As2O3 maintenance
To define the risk of relapse for patients on oral As2O3 maintenance, the impact of gender, age (> 40 years), hemoglobin (< 10 g/dL), presentation WBC count (> 10 × 109/L), presentation platelet count (< 40 × 109/L), history of APL differentiation syndrome, peak WBC count during treatment (> 10 × 109/L), presence of second malignancies either before or after the diagnosis of APL, and types of maintenance regimen (regimen A vs AA vs AAA) on relapse was analyzed. The risk of relapse in females was significantly lower than that in males (P = .02, Fisher exact test). Other parameters analyzed had no significant impact on relapse rates (Table 4).
Impacts of clinicopathologic features on relapse in 76 APL patients on oral As2O3 maintenance
| Clinicopathologic parameters . | Outcome . | P . | |
|---|---|---|---|
| Remission . | Relapse . | ||
| Sex | |||
| Male | 28 | 7 | |
| Female | 40 | 1 | .02 |
| Age, y | |||
| ≤ 40 | 24 | 2 | |
| > 40 | 44 | 6 | NS (.70) |
| Presentation WBC count | |||
| ≤ 10 × 109/L | 60 | 6 | |
| > 10 × 109/L | 8 | 2 | NS (.28) |
| Presentation platelet count | |||
| < 40 × 109/L | 46 | 5 | |
| ≥ 40 × 109/L | 22 | 3 | NS (1.0) |
| APL differentiation syndrome | |||
| Present | 13 | 3 | |
| Absent | 55 | 5 | NS (.35) |
| Peak WBC count | |||
| ≤ 10 × 109/L | 28 | 3 | |
| > 10 × 109/L | 30 | 5 | NS (.46) |
| Maintenance regimens | |||
| A | 17 | 3 | |
| AA | 16 | 3 | |
| AAA | 35 | 2 | NS (.37) |
| Second cancers (before/after maintenance) | |||
| Present | 13 | 1 | |
| Absent | 35 | 7 | NS (1.0) |
| Clinicopathologic parameters . | Outcome . | P . | |
|---|---|---|---|
| Remission . | Relapse . | ||
| Sex | |||
| Male | 28 | 7 | |
| Female | 40 | 1 | .02 |
| Age, y | |||
| ≤ 40 | 24 | 2 | |
| > 40 | 44 | 6 | NS (.70) |
| Presentation WBC count | |||
| ≤ 10 × 109/L | 60 | 6 | |
| > 10 × 109/L | 8 | 2 | NS (.28) |
| Presentation platelet count | |||
| < 40 × 109/L | 46 | 5 | |
| ≥ 40 × 109/L | 22 | 3 | NS (1.0) |
| APL differentiation syndrome | |||
| Present | 13 | 3 | |
| Absent | 55 | 5 | NS (.35) |
| Peak WBC count | |||
| ≤ 10 × 109/L | 28 | 3 | |
| > 10 × 109/L | 30 | 5 | NS (.46) |
| Maintenance regimens | |||
| A | 17 | 3 | |
| AA | 16 | 3 | |
| AAA | 35 | 2 | NS (.37) |
| Second cancers (before/after maintenance) | |||
| Present | 13 | 1 | |
| Absent | 35 | 7 | NS (1.0) |
Deaths
Eight patients died during the study period. Only 2 deaths were related to APL (UPN 5 died of R3 at 100 months, UPN 19 died of R3 at 37 months; Table 3). The other 6 deaths occurred to patients while the APL was still in remission. Two deaths were vascular events (pulmonary embolism and a stroke). Four deaths resulted from unrelated cancers, 1 of which was a breast cancer that existed before APL was diagnosed (Table 3).
Survivals
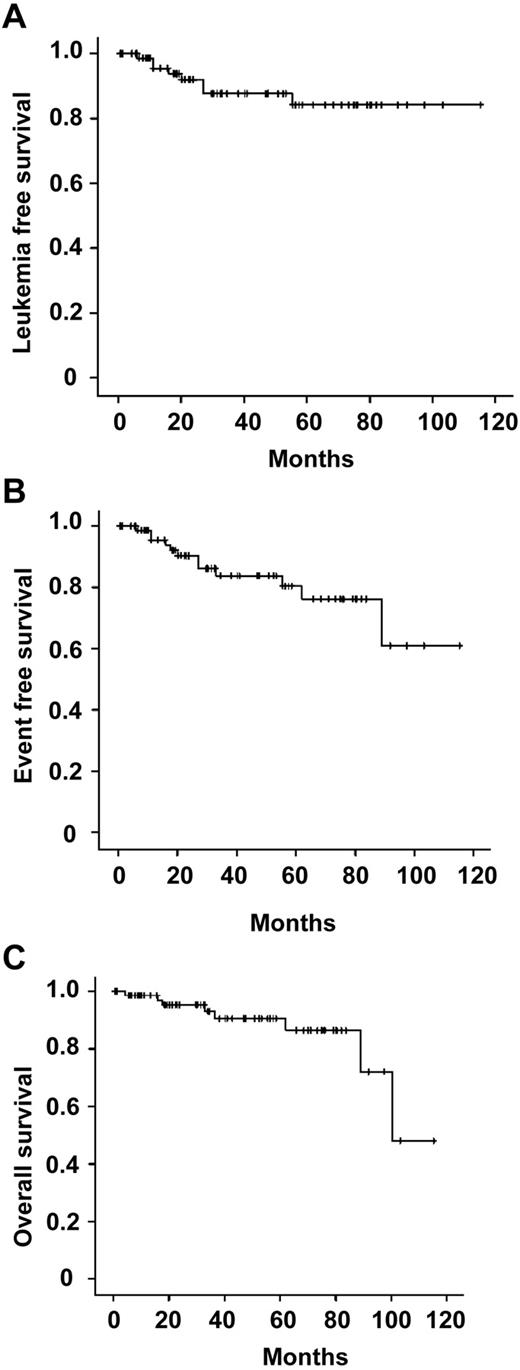
The 3-year LFS, EFS, and OS were 87.7%, 83.7%, and 90.6%, respectively (Figure 1). The impact of gender, age (≤ vs > 40 years), presentation WBC count (≤ vs > 10 × 109/L), presentation platelet count (< vs ≥ 40 × 109/L), APL differentiation syndrome, ATRA-induced leukocytosis (peak WBC ≤ vs > 10 × 109/L), history of previous or subsequent solid tumors, and type of maintenance (regimen A vs AA vs AAA) on LFS, EFS, and OS were analyzed. Male gender was associated with significantly lower LFS (P = .014, log-rank; Table 5). However, because of efficient salvage treatment for relapses and the occurrence of nonleukemia-related deaths in 4 women, gender did not impact on EFS or OS. On univariate analysis, significant factors affecting OS included age > 40 years (P = .03, log-rank) and the occurrence of previous or second cancers (P < .001). On multivariate analysis, only the occurrence of other cancers (P = .004) but not age (P = .97) was associated with an inferior OS.
Survivals of 76 patients with APL in first remission receiving oral arsenic trioxide–based maintenance regimens. (A) LFS. (B) EFS. (C) OS.
Survivals of 76 patients with APL in first remission receiving oral arsenic trioxide–based maintenance regimens. (A) LFS. (B) EFS. (C) OS.
Impacts of clinicopathologic parameters on survival in 76 APL patients on oral As2O3 maintenance regimens
| Clinicopathologic parameters . | 3-y survivals, % . | |||||
|---|---|---|---|---|---|---|
| LFS . | P (log-rank) . | EFS . | P (log-rank) . | OS . | P (log-rank) . | |
| Sex | ||||||
| Male | 71 | 73 | 92 | |||
| Female | 100 | .014 | 92 | NS (.15) | 90 | NS (.85) |
| Age, y | ||||||
| ≤ 40 | 96 | 96 | 100 | |||
| > 40 | 83 | NS (.41) | 76 | NS (.10) | 89 | .03 |
| Presentation WBC count | ||||||
| ≤ 10 × 109/L | 90 | 85 | 100 | |||
| > 10 × 109/L | 77 | NS (.38) | 77 | NS (.84) | 92 | NS (.18) |
| Presentation platelet count | ||||||
| < 40 × 109/L | 88 | 86 | 96 | |||
| ≥ 40 × 109/L | 88 | NS (.61) | 77 | NS (.12) | 85 | NS (.09) |
| APL differentiation syndrome | ||||||
| Present | 78 | 69 | 92 | |||
| Absent | 91 | NS (.31) | 88 | NS (.26) | 94 | NS (.95) |
| Peak WBC count | ||||||
| ≤ 10 × 109/L | 94 | 51 | 90 | |||
| > 10 × 109/L | 80 | NS (.34) | 77 | NS (.70) | 97 | NS (.45) |
| Maintenance regimens | ||||||
| A | 85 | 80 | 90 | |||
| AA | 90 | 90 | 100 | |||
| AAA | 92 | NS (.99) | 87 | NS (.87) | 92 | NS (.19) |
| Second cancers | ||||||
| Present | 87 | 68 | 71 | |||
| Absent | 90 | NS (.84) | 87 | NS (.08) | 100 | < .001 |
| Clinicopathologic parameters . | 3-y survivals, % . | |||||
|---|---|---|---|---|---|---|
| LFS . | P (log-rank) . | EFS . | P (log-rank) . | OS . | P (log-rank) . | |
| Sex | ||||||
| Male | 71 | 73 | 92 | |||
| Female | 100 | .014 | 92 | NS (.15) | 90 | NS (.85) |
| Age, y | ||||||
| ≤ 40 | 96 | 96 | 100 | |||
| > 40 | 83 | NS (.41) | 76 | NS (.10) | 89 | .03 |
| Presentation WBC count | ||||||
| ≤ 10 × 109/L | 90 | 85 | 100 | |||
| > 10 × 109/L | 77 | NS (.38) | 77 | NS (.84) | 92 | NS (.18) |
| Presentation platelet count | ||||||
| < 40 × 109/L | 88 | 86 | 96 | |||
| ≥ 40 × 109/L | 88 | NS (.61) | 77 | NS (.12) | 85 | NS (.09) |
| APL differentiation syndrome | ||||||
| Present | 78 | 69 | 92 | |||
| Absent | 91 | NS (.31) | 88 | NS (.26) | 94 | NS (.95) |
| Peak WBC count | ||||||
| ≤ 10 × 109/L | 94 | 51 | 90 | |||
| > 10 × 109/L | 80 | NS (.34) | 77 | NS (.70) | 97 | NS (.45) |
| Maintenance regimens | ||||||
| A | 85 | 80 | 90 | |||
| AA | 90 | 90 | 100 | |||
| AAA | 92 | NS (.99) | 87 | NS (.87) | 92 | NS (.19) |
| Second cancers | ||||||
| Present | 87 | 68 | 71 | |||
| Absent | 90 | NS (.84) | 87 | NS (.08) | 100 | < .001 |
NS indicates not significant.
Discussion
Our findings demonstrated that the long-term use of As2O3 as a maintenance regimen for APL was practicable. The commonest side effect was headache, which was a problem only when ATRA was concomitantly used with As2O3. The headache can be prevented by twice-daily dosing of ATRA. Dyspepsia can be ameliorated by antacids. Reactivation of herpes zoster is a well-recognized dermatologic problem associated with arsenic.20 After observing its frequent occurrence in the initial part of the study, we have adopted the prophylactic use of acyclovir, a practice that has effectively prevented herpes zoster reactivation in the remaining patients. Five patients presented with malignancies during this 10-year period, 4 of which occurred after the diagnosis of APL. One patient with therapy-related APL secondary to a previous heavily-pretreated mantle cell lymphoma developed colon cancer. One patient with colonic cancer already had increased carinoembryonic antigen levels at the time of initial diagnosis of APL. One patient with nasopharyngeal cancer had a family history of Darier disease, a condition that predisposed to epithelial cancers. One patient with salivary gland carcinoma had a rare syndrome of congenital facial ichthyosis. Furthermore, 10 patients in our cohort already had a history of another cancer even before their APL was diagnosed. This apparent high frequency of an antecedent cancer might be related to a high median age in our cohort and the fact that we did not exclude patients with a previous history of cancer, as opposed to typical clinical trials where a previous history of cancer might be an exclusion criterion. Importantly, malignancies traditionally attributed to chronic environmental arsenic poisoning, including bladder cancer, lung cancer, and other epithelial cancers, were not seen in our patients. Finally, the total cumulative dose of oral As2O3 in our regimen was approximately 1980 mg administered over 2 years, which was comparable with the cumulative doses of intravenous As2O3 reported previously (1800 mg over 18 months,10 1630 mg over 9 months,12 and 1720 mg over 2 years13 ). None of these studies reported excessive cancers. Therefore, the apparent high frequency of cancers before and after oral As2O3 maintenance in this study could conceivably be coincidental. Our observations therefore showed that prolonged therapeutic use of As2O3 was safe and not associated with unacceptable side effects.
These results showed that arsenic maintenance was also practical. Because of the much smaller risk of cardiac arrhythmias, oral As2O3 could be taken at home, so that outpatient treatment became feasible. Because side effects were few and the A, AA, and AAA regimens were entirely oral, patients were generally compliant with treatment.
Moreover, our protocol is a much simpler approach compared with other established regimens.5-8 Patients were given ATRA and the standard 3:7 induction, 2 2:5 consolidation, followed by an oral maintenance regimen based on oral As2O3. Because only patients in remission were referred, exact data on the CR rate of all newly diagnosed cases were not available. This protocol obviated the additional administration of another anthracycline (such as idarubicin), topoisomerase II inhibitor (mitoxantrone), and intermediate-dose Ara-C (1-1.5 g/m2) as in other protocols.5-8 Furthermore, because this study commenced about a decade ago, when the role of risk-adapted treatment for APL was still not established, patients in our study were not stratified into risk groups. Therefore, our protocol did not involve the additional use in high-risk patients of idarubicin (as in PETHEMA LPA99),5 Ara-C at higher doses (as in European APL2000),6 more prolonged Ara-C and mitoxantrone (as in PETHEMA and HOVON LPA2005),7 and etoposide and thioguanine (as in GIMEMA AIDA-2000).8 In any case, some of the patients in this cohort, because of either age or medical comorbidities, would not be able to tolerate intensive chemotherapy.
Nevertheless, our simple approach involving fewer drugs in much smaller doses gave results that compared very favorably with other more complicated protocols. Our 3-year LFS and EFS were 87.7% and 83.7%, respectively, which were comparable or better than those reported in studies without risk-adapted approaches, where relapses occurred variably from 16% to > 30% of patients.14,21,22 Even with risk-adapted approaches using more intensive chemotherapeutic drugs in higher doses in high-risk persons, relapses still occurred in slightly > 10% of patients.2,3,5,7 However, a limitation of such comparisons is that published trials registered patients at diagnosis and outcome therefore included induction and consolidation, whereas our study recruited patients who were at CR1. A comparison with data from studies that had no maintenance treatment, or had ATRA maintenance alone might be more appropriate, but Kaplan-Meier curves generated from the time of maintenance were also not available from published studies. Longer follow-up will be required to define the long-term outcome of this cohort. However, our refusal to deny treatment to old patients and those with previous or on-going malignancies, which would occur in most reported trials, might conceivably contribute to a decline in survival on longer follow-up, as age and malignancies were found to be significant factors impacting survival in our cohort. Moreover, a selection bias toward patients who could achieve a CR1 was present in this study. These limitations notwithstanding, our results were consistent with observations from previous studies evaluating the early use of intravenous As2O3, which showed that As2O3 given during consolidation significantly improved survivals.14,15 Taken together, these observations showed that the administration of As2O3 at various time points in APL patients in CR1, either during consolidation or maintenance, might be another approach to improve the outcome.
APL is a potentially curable leukemia with modern treatment.2,3 Long-term therapy-related complications that curtail patient survivals are becoming important concerns. Indeed, therapy-related acute myeloid leukemia and myelodysplasia are increasingly reported in APL patients in durable remission. The frequency varied considerably, from 2%23 to 10%24 in patients in CR1. With the use of As2O3 maintenance, we have been able to economize on the use of multiple additional chemotherapeutic agents during consolidation and avoid the use of mercaptopurine and methotrexate as maintenance drugs. None of our cases developed therapy-related acute myeloid leukemia and myelodysplasia. The use of As2O3 during CR1 in APL may therefore be a means to decrease therapy-related acute myeloid leukemia and myelodysplasia in these potentially cured patients.
We have also analyzed risk factors that predisposed to relapses in our cohort. Interestingly, with the use of As2O3, apparently different adverse predictors have been observed. We were unable to show that high WBC and low platelet count, which were conventional risk factors for relapses,2,3 had any impact on LFS. Instead, we showed that male gender was the only factor associated with relapses. The pharmacokinetics of oral As2O3 did not appear to be different between men and women,17 so that the reason for the favorable impact of the female gender remains to be defined. More intriguing was the finding that patients who relapsed after prolonged As2O3 maintenance still responded excellently to reinduction treatment with As2O3. Therefore, despite the occurrence of relapses in approximately 10% of our patients and deaths because of non-APL causes, the 3-year OS of this cohort was still high at 90.6%.
Our observations generate several important leads that would have to be addressed in future studies. The best timing for the use of As2O3 in APL, whether during induction, consolidation, or maintenance, remains to be defined. Although in all previous studies intravenous As2O3 has been used, we have shown oral As2O3 to be safe and equally efficacious.15,17 Different parameters, including age, presentation WBC count and platelet count,2,3 PML-RARA status on conclusion of consolidation,4 and CD56 expression25 have been defined as risk factors using non-As2O3–containing regimens. It remains to be defined whether the early use of As2O3 in the APL treatment algorithm might overcome these conventional risk factors. Furthermore, new prognostic indicators associated with the early use of As2O3 may have to be determined, so as to further improve patient stratification for treatment.
Although As2O3 is effective as a single agent, increasing evidence indicates that, when combined with other drugs, As2O3 is more efficacious. We have used oral As2O3 in combination with ATRA and ascorbic acid. The addition of ATRA was based on the observation that ATRA plus As2O3 was more efficacious in the treatment of both newly diagnosed10,11 and relapsed APL26 compared with As2O3 alone. Our use of ascorbic acid was based empirically on its in vitro27-29 and in vivo30 synergisms with As2O3 observed in previous studies (albeit in non-APL cell lines or myeloma) and the fact that it was an innocuous vitamin. Again, it is notable that, for patients who relapsed after oral As2O3-based maintenance regimens (A, AA, or AAA), they continued to respond to AAA treatment. Hence, future studies are needed to determine whether As2O3 may be synergistically used together with other drugs and, if so, what the best drugs are.
Finally, as to the role of maintenance, a recent study examined the effects of different maintenance regimens, composed of mercaptopurine plus methotrexate, ATRA, mercaptopurine plus methotrexate alternating with ATRA, and observation alone, on treatment outcome after consolidation with a multidrug protocol.31 The results indicated that, for patients tested negative for PML-RARA after consolidation, no significant differences in outcome existed between any of the maintenance regimens and observation. However, it must be noted that the 12-year EFS of this cohort was only 69%, meaning that improvement would still be needed. Another earlier study also reported poorer results after the use of intensive maintenance treatment (including Ara-C, daunorubicin, mitoxantrone, vindesine, etoposide, and mercaptopurine) for APL patients in PML-RARA-negative CR1.32 In retrospect, it seems apparent that such intensive maintenance would be expected to have negative impacts on outcome. Therefore, the role of As2O3-based maintenance regimens in conjunction with intensive consolidation should be further investigated.
In conclusion, our study showed that the prolonged use of As2O3 in maintenance was feasible, safe, and beneficial. Our observations provide grounds for the generation of new hypotheses to be tested, so that As2O3 can be optimally incorporated into the treatment of APL to further improve its curability.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the S. K. Yee Medication Foundation for financial support, and the Department of Pharmacy at Queen Mary Hospital for technical support in the production of oral arsenic trioxide.
Authorship
Contribution: W.-Y.A. and Y.-L.K. conceived the study, treated the patients, and wrote and approved the manuscript; C.R.K. provided oral arsenic trioxide and approved the manuscript; and H.K.K.L., S.-Y.L., H.L., D.Y.M.Y., and J.S.M.L. treated the patients and approved the manuscript.
Conflict-of-interest disclosure: The University of Hong Kong holds a United States patent (7,521,071 B2) and a Japan patent (4786341) for the use of oral arsenic trioxide in the treatment of leukemia. W.-Y.A., C.R.K., and Y.-L.K. were employed by or associated with the University of Hong Kong. The remaining authors declare no competing financial interests.
Correspondence: Yok-Lam Kwong, Department of Medicine, Queen Mary Hospital, Pokfulam Road, Hong Kong, China; e-mail: ylkwong@hkucc.hku.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal