Abstract
Multiple myeloma (MM) patients who receive killer cell Ig–like receptor (KIR) ligand–mismatched, T cell–depleted, allogeneic transplantation may have a reduced risk of relapse compared with patients who receive KIR ligand–matched grafts, suggesting the importance of this signaling axis in the natural killer (NK) cell-versus-MM effect. Expanding on this concept, IPH2101 (1-7F9), an anti-inhibitory KIR mAb, enhances NK-cell function against autologous MM cells by blocking the engagement of inhibitory KIR with cognate ligands, promoting immune complex formation and NK-cell cytotoxicity specifically against MM cell targets but not normal cells. IPH2101 prevents negative regulatory signals by inhibitory KIR, whereas lenalidomide augments NK-cell function and also appears to up-regulate ligands for activating NK-cell receptors on MM cells. Lenalidomide and a murine anti-inhibitory NK-cell receptor Ab mediate in vivo rejection of a lenalidomide-resistant tumor. These mechanistic, preclinical data support the use of a combination of IPH2101 and lenalidomide in a phase 2 trial for MM.
Introduction
Natural killer (NK) cells exert cytotoxicity against multiple myeloma (MM), and some therapies for MM appear to recover or enhance NK-cell function against MM.1-5 Lenalidomide in particular confers NK-cell expansion and activation associated with tumor cell apoptosis.4,5 MM cells up-regulate the expression of ligands to NK cell–inhibitory killer cell immunoglobulin–like receptor (KIR)6 and KIR-ligand mismatch in T cell–depleted, allogeneic stem cell transplantation may reduce the risk of relapse in MM patients, suggesting that this signaling axis may be particularly important.7
IPH2101 is a human IgG4 mAb against common inhibitory KIR2DL-1, KIR2DL-2, and KIR2DL-3.8 IPH2101 enhances NK-cell function against malignant cells by preventing inhibitory KIR-ligand interaction and subsequent inhibitory signaling.8
In the present study, we provide novel data characterizing mechanisms by which inhibitory KIR blockade augments NK-cell function against MM, sparing normal cells. In addition, we uncover novel immunomodulatory properties of lenalidomide that likely contribute to enhanced NK-cell activity. We demonstrate that a murine anti-inhibitory NK-cell receptor Ab and lenalidomide further augment NK-cell function against MM compared with either agent alone, leading to in vivo rejection of a lenalidomide-resistant tumor. These data support the initiation of a steroid-sparing, phase 2 trial of IPH2101 and lenalidomide in MM.
Methods
Cells
Cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS (ICN Biomedicals) at 37° in 5% CO2. NK cells and PBMCs from healthy donors (American Red Cross, Columbus, OH, and Indiana Blood Center, Indianapolis, IN) and PBMCs and BM aspirates from patients with MM obtained per Institutional Review Board–approved protocols were prepared as described previously.9 The MM cell lines U266 and K562 were from the American Type Culture Collection. We were unable to procure sufficient patient blood volume to enrich for NK cells from MM patient donors; therefore, experiments using patient-derived NK cells were conducted in PBMCs at effector:target (E:T) ratios based on the proportion of CD56+, CD3− NK cells in patient PBMCs determined by flow cytometry.
Abs and reagents
IPH2101 (and PE-labeled anti-IPH2101) were provided by Innate Pharma. Lenalidomide was from Toronto Research Chemicals and John C. Byrd (The Ohio State University, Columbus, OH). Flow Abs were from Beckman Coulter, BD Pharmingen, eBioscience, R&D Systems, and Miltenyi Biotec. NKG2D-blocking Ab was from BioLegend. Abs against TRAIL, DNAM-1, and HLA class I (and isotypes) were from BD Biosciences, and 7-amino-actinomycin D was from Sigma-Aldrich.
Antigen expression assays
U266 cells were stained with 7-amino-actinomycin D and PE-Ab, incubated at 4°C for 15 minutes, and washed with MACS buffer. Ten thousand cells and QuantiBRITE PE beads (BD Biosciences) were collected with a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo Version 7.6.1 software (TreeStar). The median PE-relative fluorescence intensity (RFI) measurements were converted to number of PE Abs bound per cell using the QuantiBRITE PE beads. Expression of activating ligands on CD138+ MM cells was assessed at baseline and after 24 hours in lenalidomide (5nM) and on U266 cells after 5 days in lenalidomide (5μm).
Immune complex assays
Immune complex formation between patient PBMCs and autologous CD138+ MM cells or CD138− BM cells was examined using a 2-color flow cytometric technique described previously.9 Effector cells (IPH2101- or control-treated) were labeled with CFSE (Sigma-Aldrich) and target cells were labeled with Paul Karl Horan dye (PKH; Sigma-Aldrich). Data were collected using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest Version 4.0 software (BD Biosciences).
ELISPOT assays
Europium-release cytotoxicity assays
Cytotoxicity was analyzed in europium-release assays per the manufacturer's instructions (DELFIA EuTDA Cytotoxicity kit; PerkinElmer). Specific cytotoxicity was calculated as follows: % cytotoxicity = (experimental release − spontaneous release) × 100/(maximal release − spontaneous release). Effectors incubated with or without 30 μg/mL of IPH-2101 for 30 minutes at 4°C and 10μM lenalidomide (or DMSO) were cocultured with DELFIA BATDA–labeled targets. In blocking experiments, NK cells were first incubated with 80 μg/mL of NKG2D, 40 μg/mL of DNAM-1, and 50 μg/mL of TRAIL mAbs or 170 μg/mL of isotype for 30 minutes at 4°C.
Flow cytometric killing assay
Flow cytometric cytotoxicity assays were conducted as described previously.9,11 Patient-derived PBMCs were incubated for 72 hours in control conditions or with lenalidomide and/or IPH2101. Data were collected on an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo Version 7.6.1 software.
In vivo tumor cell rejection
The in vivo tumor cell rejection method was described previously.12 RMA (H-2b) is resistant to lenalidomide-induced apoptosis. GFP+-RMA cells were generated by lentivirus transduction with TRIpdU3FE1aEGFP. Spleen cells stained with 5μM PKH26 (Sigma-Aldrich) and mixed with GFP+-RMA cells (ratio 1:1) were injected intravenously into C57BL/6J mice (Charles River Laboratories). Forty hours later, liver mononuclear cells were separated by Ficoll gradient 80%/40%. Survival of engrafted cell populations was analyzed by flow cytometry. Results are presented as ratio of GFP+-RMA to spleen cells.
BM NK-cell CD107a expression
Freshly isolated, whole BM aspirates from patients with MM (n = 5) were incubated in control conditions or with IPH2101 (30 μg/mL) and/or lenalidomide (5μM) for 24 hours. The percentage of NK cells identified as CD56+CD16+CD3−C107a+ in the lymphocyte gate by flow cytometry was determined for each condition.
Statistical analysis
Means and SDs from experiments with 2 conditions were analyzed with the Student t test. ANOVA was used to analyze experiments with multiple conditions with planned comparisons conducted using the method of Bonferroni. Statistical significance was determined by P < .05.
Results and discussion
IPH2101 and lenalidomide enhance NK-cell function against MM
IPH2101 increased NK-cell cytotoxicity against U266 cells (Figure 1A all pairwise comparisons, P < .05) and patient-derived NK cells against primary MM targets (Figure 1B right panel, P = .008). Interestingly, blocking either inhibitory KIR on NK cells (with IPH2101) or blocking HLA class I on U266 MM tumor cells enhanced killing, but there was no additive effect when both inhibitory KIR and HLA class I were blocked (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
IPH2101 increases NK-cell recognition of and cytotoxicity against MM targets. (A) IPH2101 (30 μg/mL) augmented NK cell–mediated killing of U266 MM cells measured by a target-based, europium-release cytotoxicity assay. At E:T ratios of 2.5:1, 5:1, and 10:1, IPH2101 increased cytotoxicity by an average of 1.76 ± 0.28-fold, 1.4 ± 0.09-fold, and 1.48 ± 0.08-fold relative to control, respectively (P < .05 for all comparisons, results represent n = 10 independent donor experiments). (B) In a complementary manner, NK-cell production of granzyme B (GrB) measured by ELISPOT assay served as an effector-cell–based killing assay. IPH2101 enhanced NK-cell GrB production against U266 targets (E:T 20:1, left, isotype control mean 139 ± 63 vs IPH2101 treated 348 ± 20, n = 3 independent experiments, P = .005). IPH2101 also enhanced patient-derived NK-cell GrB secretion against primary MM cell targets (right, E:T 10:1, control mean 59 ± 2.3 vs IPH2101 treated 103 ± 4.6). *P = .008, results represent n = 5 independent donor experiments. The black bar in each graph represents a positive control for the assay using effector cells versus the K562 cell line. (C) Pretreatment of NK cells with IPH2101 significantly increased the number of immune complexes observed between patient-derived effector cells and purified, autologous CD138+ MM cells (E:T 1:1, control mean 751 ± SD 181 vs IPH2101 treated 1225 ± 207, P = .041), but not against the residual CD138− normal BM cells (E:T 1:1, control mean 458 ± 208 vs IPH2101 treated 507 ± 218, P = nonsignificant, n = 3 independent donor experiments). (D) The increase in immune complexes was associated with enhanced NK-cell lysis of autologous CD138+ MM cell targets (control 42% ± 8% vs IPH2101 treated 63% ± 12%, *P = .0135), but no enhancement of cytotoxicity against CD138− autologous, normal BM elements (control 6% ± 5% vs IPH2101 treated 3% ± 2%, P = nonsignificant, E:T = 20:1, n = 3 independent donor experiments). (E) Left, freshly isolated, healthy donor NK cells were pretreated with lenalidomide (5μM), IPH2101 (30 μg/mL), or the combination, and IFN-γ production was measured by ELISPOT assay against primary MM cells in coculture for 4 hours. Lenalidomide (mean 609 spots/well ± 34) and IPH2101 (568 ± 20) each increased NK-cell IFN-γ production beyond control conditions (483 ± 15, *P < .05). NK cells pretreated with both lenalidomide and IPH2101 (807 ± 50) led to the greatest IFN-γ production of any condition, and the interaction was statistically significant, suggesting these agents induced a synergistic effect (**P < .0182, n = 3 independent donor experiments, similar results were obtained with NK cells vs U266 MM targets, data not shown). Right, patient-derived NK cells against autologous MM tumor cells. Lenalidomide (**mean 74 ± 9 spots/well), IPH2101 (***mean 44 ± 4 spots/well), and the combination (*mean 99 ± 7 spots/well) all enhanced IFN-γ production beyond control (20 ± 2) conditions, overall P < .0001, P < .05 for each pairwise comparison shown, representative findings of n = 3 independent experiments. (F) PBMCs incubated in control conditions or lenalidomide (10μM) and/or IPH2101 (30 μg/mL) served as effector cells against U266 MM cell targets. At an E:T ratio of 30:1, lenalidomide increased cytotoxicity as measured by specific release by 1.39 ± 0.10-fold relative to control (P < .01), IPH2101 increased the specific release by 1.48 ± 0.21-fold (P < .01), whereas the combination of lenalidomide and IPH2101 increased the specific release by 2.09 ± 0.21-fold relative to control (P < .001), which was also significantly greater than the cytotoxicity observed with either lenalidomide or IPH-2010 alone (P < .01 for each comparison). Similar results were obtained using at an E:T ratio of 60:1 (right). (G) Patient-derived NK-cell cytotoxicity against autologous MM target cells was enhanced by lenalidomide (mean 110 ± 8 spots/well), IPH2101 (106 ± 11), or the combination (128 ± 9) compared with control conditions (81 ± 7), overall ANOVA P = .0001, all pairwise, planned comparisons shown P < .05, representative data of n = 3 independent experiments. (H) Whole BM aspirates obtained from patients with MM (n = 5) were incubated with or without IPH2101 (30 μg/mL) and/or lenalidomide (5μM) for 24 hours. The percentage of NK cells expressing CD107a increased in response to lenalidomide and IPH2101 (20% ± 6%) compared with control (11.3% ± 2%, P < .05).
IPH2101 increases NK-cell recognition of and cytotoxicity against MM targets. (A) IPH2101 (30 μg/mL) augmented NK cell–mediated killing of U266 MM cells measured by a target-based, europium-release cytotoxicity assay. At E:T ratios of 2.5:1, 5:1, and 10:1, IPH2101 increased cytotoxicity by an average of 1.76 ± 0.28-fold, 1.4 ± 0.09-fold, and 1.48 ± 0.08-fold relative to control, respectively (P < .05 for all comparisons, results represent n = 10 independent donor experiments). (B) In a complementary manner, NK-cell production of granzyme B (GrB) measured by ELISPOT assay served as an effector-cell–based killing assay. IPH2101 enhanced NK-cell GrB production against U266 targets (E:T 20:1, left, isotype control mean 139 ± 63 vs IPH2101 treated 348 ± 20, n = 3 independent experiments, P = .005). IPH2101 also enhanced patient-derived NK-cell GrB secretion against primary MM cell targets (right, E:T 10:1, control mean 59 ± 2.3 vs IPH2101 treated 103 ± 4.6). *P = .008, results represent n = 5 independent donor experiments. The black bar in each graph represents a positive control for the assay using effector cells versus the K562 cell line. (C) Pretreatment of NK cells with IPH2101 significantly increased the number of immune complexes observed between patient-derived effector cells and purified, autologous CD138+ MM cells (E:T 1:1, control mean 751 ± SD 181 vs IPH2101 treated 1225 ± 207, P = .041), but not against the residual CD138− normal BM cells (E:T 1:1, control mean 458 ± 208 vs IPH2101 treated 507 ± 218, P = nonsignificant, n = 3 independent donor experiments). (D) The increase in immune complexes was associated with enhanced NK-cell lysis of autologous CD138+ MM cell targets (control 42% ± 8% vs IPH2101 treated 63% ± 12%, *P = .0135), but no enhancement of cytotoxicity against CD138− autologous, normal BM elements (control 6% ± 5% vs IPH2101 treated 3% ± 2%, P = nonsignificant, E:T = 20:1, n = 3 independent donor experiments). (E) Left, freshly isolated, healthy donor NK cells were pretreated with lenalidomide (5μM), IPH2101 (30 μg/mL), or the combination, and IFN-γ production was measured by ELISPOT assay against primary MM cells in coculture for 4 hours. Lenalidomide (mean 609 spots/well ± 34) and IPH2101 (568 ± 20) each increased NK-cell IFN-γ production beyond control conditions (483 ± 15, *P < .05). NK cells pretreated with both lenalidomide and IPH2101 (807 ± 50) led to the greatest IFN-γ production of any condition, and the interaction was statistically significant, suggesting these agents induced a synergistic effect (**P < .0182, n = 3 independent donor experiments, similar results were obtained with NK cells vs U266 MM targets, data not shown). Right, patient-derived NK cells against autologous MM tumor cells. Lenalidomide (**mean 74 ± 9 spots/well), IPH2101 (***mean 44 ± 4 spots/well), and the combination (*mean 99 ± 7 spots/well) all enhanced IFN-γ production beyond control (20 ± 2) conditions, overall P < .0001, P < .05 for each pairwise comparison shown, representative findings of n = 3 independent experiments. (F) PBMCs incubated in control conditions or lenalidomide (10μM) and/or IPH2101 (30 μg/mL) served as effector cells against U266 MM cell targets. At an E:T ratio of 30:1, lenalidomide increased cytotoxicity as measured by specific release by 1.39 ± 0.10-fold relative to control (P < .01), IPH2101 increased the specific release by 1.48 ± 0.21-fold (P < .01), whereas the combination of lenalidomide and IPH2101 increased the specific release by 2.09 ± 0.21-fold relative to control (P < .001), which was also significantly greater than the cytotoxicity observed with either lenalidomide or IPH-2010 alone (P < .01 for each comparison). Similar results were obtained using at an E:T ratio of 60:1 (right). (G) Patient-derived NK-cell cytotoxicity against autologous MM target cells was enhanced by lenalidomide (mean 110 ± 8 spots/well), IPH2101 (106 ± 11), or the combination (128 ± 9) compared with control conditions (81 ± 7), overall ANOVA P = .0001, all pairwise, planned comparisons shown P < .05, representative data of n = 3 independent experiments. (H) Whole BM aspirates obtained from patients with MM (n = 5) were incubated with or without IPH2101 (30 μg/mL) and/or lenalidomide (5μM) for 24 hours. The percentage of NK cells expressing CD107a increased in response to lenalidomide and IPH2101 (20% ± 6%) compared with control (11.3% ± 2%, P < .05).
We then studied these effects in the autologous setting using patient-derived, effector NK cells against autologous MM tumor cell and normal cell targets. We verified IPH2101 binding on NK cells and HLA class I expression on MM tumor cells (supplemental Figure 2). By preventing inhibitory KIR-ligand interaction, IPH2101 enhanced immune complex formation against autologous MM cells (Figure 1C; E:T = 1:1, control-treated mean 751 ± SD 181 vs IPH2101-treated 1225 ± 207, P = .041) but not against residual CD138− normal BM cells. Similarly, IPH2101 preferentially increased NK-cell lysis of autologous MM targets (Figure 1D; E:T = 20:1, control-treated cytotoxicity = 42% ± 8% vs IPH2101-treated = 63% ± 12%, P = .0135), but no increase in cytotoxicity was observed against CD138− normal BM elements.
Lenalidomide and IPH2101 also increased NK-cell IFN-γ production (Figure 1E left panel shows enriched healthy donor NK cells vs primary MM-cell targets, right panel shows patient-derived NK cells vs autologous MM-cell targets), PBMC cytotoxicity against U266 MM cell line targets (Figure 1F), and patient-derived NK-cell cytotoxicity against autologous MM targets (Figure 1G). Furthermore, in freshly isolated, whole BM aspirates from patients with MM, IPH2101 and lenalidomide altered expression of CD107a on NK cells in BM (P = .0214). Planned comparisons revealed that the percentage of NK cells expressing CD107a increased in BM incubated in the combination of IPH2101 and lenalidomide by 1.81 ± 0.5-fold (P < .05) relative to control conditions (Figure 1H). In these experiments, NK cells comprised 4.1% (± SD 1.7) of BM cells, and this proportion was consistent across conditions.
Lenalidomide modulates NK-cell ligand expression on MM cells
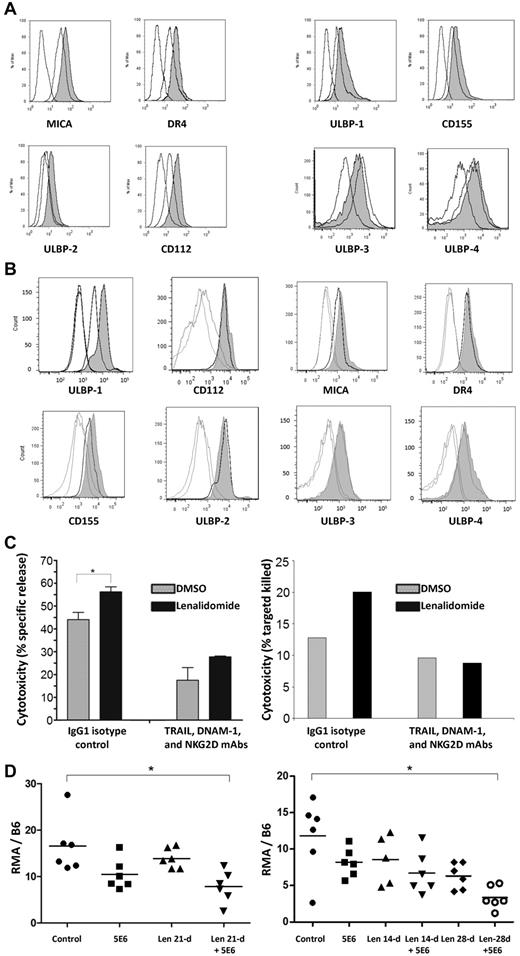
The expression of MICA (increased 73.6% ± 26.6%), DR4 (increased 95.6% ± 9.5%), ULBP-2 (increased 86.1% ± 16.1%), and CD112 (increased 114% ± 6.9%) were enhanced on U266 cells by 5 days of incubation in lenalidomide (5μM) as shown in Figure 2A left panel (Figure 2A right panel shows that the expression of ULBP-1, CD155, ULBP-3, and ULBP-4 was not affected). In primary MM cells, ULBP-1 expression was consistently increased (153% ± 24%, P = .016 compared with control-treated cells, Figure 2B), whereas the expression of other activating ligands was not reproducibly enhanced in any of the cases examined.
Lenalidomide modulates MM expression of NK cell–activating ligands and in combination with IPH2101 enhances in vivo tumor-cell rejection. (A) Incubation of U266 MM cells in lenalidomide (5μM) for 5 days led to alteration of NK cell–ligand expression, representative results shown for each ligand (open curves are isotype and control, shaded curve is lenalidomide treated). The average Ab-binding capacity of MICA, DR4, ULBP2, and CD112 increased by 73.6% ± 26.6%, 95.6% ± 9.5%, 86.1% ± 16.1%, and 114% ± 6.9%, respectively, compared with control cells (P < .05 for all comparisons, left). The expression of ULBP-1, CD155, ULBP-3, and ULBP-4 was not affected (right). (B) A similar effect was observed in primary MM cells (n = 7) all of which expressed ULBP-1 at baseline; a representative result is shown. Lenalidomide (5nM) for 24 hours increased the expression of ULBP-1 by 153% ± 24% (P = .016), but did not reproducibly alter expression of any of the other ligands shown in all cases examined. (C) NK-cell lysis of U266 cells was augmented 1.22 ± 0.05-fold relative to control (P < .05) when targets were pretreated with lenalidomide to increase activating ligand expression as shown in panel A. However, this effect was lost when NK cells were preincubated in blocking Abs against NKG2D, DNAM-1, and TRAIL, suggesting functional relevance to lenalidomide's modulation of activating ligands on MM target cells. A similar effect was observed in patient-derived NK cells against autologous MM-cell targets (right; n = 2 independent experiments). (D) A single 5-μg dose of 5E6 led to a slight reduction of observed RMA cells, and pretreatment of animals with lenalidomide for 21 days enhanced this effect (control mean 16.5 ± 5.9 vs 5E6 plus lenalidomide, 7.8 ± 3.5, P < .01). Similar results were observed with 5E6 (10 μg) with lenalidomide at 14 or 28 days (E). The combination of 5E6 and lenalidomide significantly reduced tumor burden (11.8 ± 5.1 for control vs 3.4 ± 1.6 for 5E6 plus lenalidomide, P < .005).
Lenalidomide modulates MM expression of NK cell–activating ligands and in combination with IPH2101 enhances in vivo tumor-cell rejection. (A) Incubation of U266 MM cells in lenalidomide (5μM) for 5 days led to alteration of NK cell–ligand expression, representative results shown for each ligand (open curves are isotype and control, shaded curve is lenalidomide treated). The average Ab-binding capacity of MICA, DR4, ULBP2, and CD112 increased by 73.6% ± 26.6%, 95.6% ± 9.5%, 86.1% ± 16.1%, and 114% ± 6.9%, respectively, compared with control cells (P < .05 for all comparisons, left). The expression of ULBP-1, CD155, ULBP-3, and ULBP-4 was not affected (right). (B) A similar effect was observed in primary MM cells (n = 7) all of which expressed ULBP-1 at baseline; a representative result is shown. Lenalidomide (5nM) for 24 hours increased the expression of ULBP-1 by 153% ± 24% (P = .016), but did not reproducibly alter expression of any of the other ligands shown in all cases examined. (C) NK-cell lysis of U266 cells was augmented 1.22 ± 0.05-fold relative to control (P < .05) when targets were pretreated with lenalidomide to increase activating ligand expression as shown in panel A. However, this effect was lost when NK cells were preincubated in blocking Abs against NKG2D, DNAM-1, and TRAIL, suggesting functional relevance to lenalidomide's modulation of activating ligands on MM target cells. A similar effect was observed in patient-derived NK cells against autologous MM-cell targets (right; n = 2 independent experiments). (D) A single 5-μg dose of 5E6 led to a slight reduction of observed RMA cells, and pretreatment of animals with lenalidomide for 21 days enhanced this effect (control mean 16.5 ± 5.9 vs 5E6 plus lenalidomide, 7.8 ± 3.5, P < .01). Similar results were observed with 5E6 (10 μg) with lenalidomide at 14 or 28 days (E). The combination of 5E6 and lenalidomide significantly reduced tumor burden (11.8 ± 5.1 for control vs 3.4 ± 1.6 for 5E6 plus lenalidomide, P < .005).
To test the functional consequence of these effects, the cytotoxicity of NK cells incubated in isotype control mAbs or a mixture of anti-TRAIL, anti–DNAM-1, and anti-NKG2D blocking mAbs was measured against MM-cell targets pretreated in DMSO or lenalidomide. NK-cell cytotoxicity was enhanced 1.22 ± 0.05-fold (Figure 2C left panel, P < .05) against U266 cells pretreated with lenalidomide; however, this effect was lost when NK cells were preincubated in a mixture of anti-TRAIL, anti–DNAM-1, and anti-NKG2D blocking Abs. A similar effect was observed in patient-derived NK cells against autologous MM targets (Figure 2C right panel shows representative finding of n = 2 independent experiments).
Lenalidomide and a murine anti-inhibitory NK-cell receptor Ab mediate in vivo rejection of a lenalidomide-resistant tumor
Mice received lenalidomide (25 mg/kg/d) for 14, 21, or 28 days. One day before inoculation with RMA tumor cells, mice were treated with a murine Ab [anti-Ly49C/I F(ab′)2 or “5E6”], equivalent to the anti-KIR Ab in humans at suboptimal doses of 5 or 10 μg. After 40 hours, the combination of 5E6 and lenalidomide led to significantly reduced tumor burden (Figure 2D-E). These results suggest at least an additive in vivo effect of mAb-mediated inhibitory NK-cell receptor blockade and lenalidomide in combination. Because the RMA cell line is resistant to direct effects of lenalidomide, these findings suggest the immunomodulatory properties of the agent combined with 5E6 to enhance NK cell–mediated tumor cell in vivo clearance.
The therapeutic potential of KIR-ligand interruption was first demonstrated in haplo-identical, T cell–depleted, allogeneic stem cell transplantation in which donor-derived, alloreactive NK cells could lyse recipient tumor cells.13-15 Infusion of haploidentical, KIR-ligand–mismatched NK cells to relapsed/refractory MM patients is associated with a 50% response rate after reduced-intensity conditioning in the autograft setting, and KIR-ligand mismatch may contribute to the reduced incidence of relapse in the setting of T cell–depleted allografting as well.7,16
Whether an NK cell lyses a target cell depends on signaling via activating and inhibitory receptors interacting with cognate ligands on the surface of candidate targets.17 NK cells must express DNAM-1, NKG2D, or NKp46 to kill MM cells.18 However, every cytolytic NK cell must express at least one inhibitory KIR ligand, which are present in greater numbers and appear to bind with greater avidity than activating KIR ligands.19,20 Therefore, even in the presence of activating receptor-ligand interaction, NK cells may be prevented from initiating cytotoxicity if a concomitant inhibitory KIR-ligand interaction is present.19,20 Therefore, IPH2101 provides release from inhibition, whereas lenalidomide simultaneously promotes NK-cell expansion and activation and appears to induce favorable modulation of activating ligands on MM targets. That a murine anti-inhibitory NK-cell receptor Ab and lenalidomide mediate in vivo rejection of a lenalidomide-resistant tumor suggests that this effect was due primarily to favorable immunomodulatory effects.
A recent phase 3 trial demonstrated superior survival in MM patients receiving lenalidomide with “low-dose” dexamethasone (40 mg weekly) compared with “high-dose” dexamethasone (40 mg/d on days 1-4, 9-12, and 17-20 of a 28-day cycle).21 Recent studies demonstrate that dexamethasone also profoundly impairs the immunomodulatory profile of lenalidomide.22,23 These findings have fostered interest in developing steroid-limiting or steroid-sparing treatments to maximize the immunomodulating properties of lenalidomide.24 Our data provide a preclinical justification for a phase 2 trial of IPH2101 in combination with lenalidomide as the first “dual immunotherapy” for patients with MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a Conquer Cancer Foundation Career Development Award (60019229 to D.M.B.), a Multiple Myeloma Opportunities for Research and Education grant (to D.M.B.), and a Pelotonia Idea grant (to D.M.B.).
Authorship
Contribution: D.M.B. designed and performed the research and wrote the manuscript; C.E.B., S.Z., S.M.C., S.S., M.B., C.B., J.L., and D.C. designed and performed the research; P.A. and F.R. designed the research and contributed vital reagents; J.Z. analyzed the data; C.C.H. and Y.E. designed the research; M.A.C. designed the research and assisted in writing the manuscript; and S.S.F. designed the research and assisted in writing the manuscript.
Conflict-of-interest disclosure: D.M.B. and S.S.F. have received research funding from Innate Pharma. P.A., F.R., M.B., and C.B. are employees of Innate Pharma. The remaining authors declare no competing financial interests.
Correspondence: Don M. Benson Jr, MD, PhD, B-310 Starling Loving Hall, 320 W 10th Ave, Columbus OH 43210-1240; e-mail: don.benson@osumc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal