Abstract
Cervical internal carotid artery (cICA) occlusion is a recognized cause of acute ischemic stroke (AIS) in sickle cell disease (SCD), but the associated clinical and radiologic features are not well described. We reviewed data on cervical magnetic resonance angiography (cMRA) performed prospectively in 67 patients (55 children) for indications including transcranial Doppler (TCD) abnormalities, AIS, or previous AIS. cICA lesions were seen in 10 (15%) patients, including 4 of 7 patients presenting with AIS, and appear to have been missed on first presentation in 4 of 10 patients with previous AIS. Radiologic features in 7 patients were consistent with dissection. In 2 patients, there was strong clinical and radiologic evidence for thromboembolic AIS, and this was also considered possible in 4 other patients. Three of the 4 AIS patients were anticoagulated acutely, and the nontreated patient had recurrent, probably thromboembolic, AIS. TCD findings were variable, but in 4 patients there were high velocities in the cerebral vessels contralateral to the cICA stenosis. We suggest that all patients with AIS should have cMRA during acute evaluation to identify cICA occlusions that may require anticoagulation. Routine screening of children with SCD should also include evaluation of neck vessels by carotid Doppler followed by cMRA if a cervical vascular lesion is suspected.
Introduction
Previous studies have reported on intracranial angiography in patients with sickle cell disease (SCD) and correlated findings with transcranial Doppler (TCD) abnormalities.1-5 There are several reports of arterial ischemic stroke (AIS) associated with lesions in the cervical internal carotid artery (cICA) detected at autopsy2 or using digital subtraction angiography,6,7 but the spectrum of radiologic features and clinical consequences have not been well described, at least in part because magnetic resonance angiography (MRA) and TCD are usually restricted to the major intracerebral vessels.3-5,8-10
Recently, cervical arterial lesions have been reported in children with SCD undergoing cervical Doppler scanning.11,12 After observing 3 patients presenting acutely with stroke and cICA occlusion, we decided to include cervical MRA (cMRA) in our routine neuroimaging protocol. We present 10 patients with cICA occlusion or stenosis and highlight clinical implications.
Methods
Over the past 4 years, all children and adults with SCD presenting to our clinic with acute neurologic events, TCD abnormalities, or previous history of stroke were scanned to a standardized protocol on a 1.5 T scanner (General Electric). T2-weighted fast-spin echo, fluid attenuated inversion recovery, and diffusion sequences (b-1000) were acquired in the axial plane with a sagittal T1-weighted spin-echo sequence. A 3D time-of-flight multislab MRA (noncontrasted) was obtained of the intracranial vessels and neck vessels. The MRA images were reconstructed as rotational maximum-intensity projections in 2 planes. All MRA images were also viewed as the axial unreconstructed images.
Magnetic resonance imaging (MRI) and MRA images were initially read by a neuroradiologist (J.E.) who was aware of the clinical scenario from clinical details on the MRI request. They were reviewed in regular clinical meetings with the clinicians involved in their care (P.T., C.H., C.A., N.G., and F.K.). Positive images were then independently reported by a second neuroradiologist (P.B.) who had no knowledge of the clinical scenario. Any discrepancies in interpretation were resolved between the 2 radiologists. The degree of cICA stenosis was graded subjectively according to accepted nomenclature as mild (< 50%), moderate (50%-70%), or severe (70%-99%)13
This study was registered as an audit with our hospital's clinical effectiveness unit (registration number 10/83) and informed consent was not required from patients contributing data.
Results
MRA scans
Eighty scans from 67 patients (29 males; 44%) were reviewed. Fifty-eight patients were scanned once, 5 twice, and 4 were scanned 3 times. Median age at first scan was 9 years (range, 3.4-44.2). Indications for MR scanning and numbers in each category with cICA lesions are shown in Table 1. MR findings are presented in Table 2 and in Figures 1,Figure 2–3. Ten of 67 (15%) patients had cICA occlusions, including 3 of 12 adults (> 16 years of age). There were no discrepancies between the 2 study radiologists in the interpretation of these scans. Other cMRA abnormalities included tortuosity of cICA and vertebral arteries and prominent, tortuous anterior spinal artery (Figure 3A). We did not observe any stenotic or occlusive lesions in the vertebral arteries.
Indications for MR scan and prevalence of cICA occlusion or stenosis
| Primary indication for MR scan . | No. scanned . | No. (%) with cICA lesions . |
|---|---|---|
| Abnormal TCD | 18 | 1 (6) |
| Conditional TCD | 13 | 1 (8) |
| Inadequate TCD | 11 | 0 (0) |
| Low-velocity/asymmetric TCD | 2 | 0 (0) |
| Acute stroke | 7 | 4 (57) |
| Previous stroke | 10 | 4 (40) |
| Other neurological symptoms* | 4 | 0 (0) |
| Pre-BM transplantation | 2 | 0 (0) |
| Total | 67 | 10 (15) |
| Primary indication for MR scan . | No. scanned . | No. (%) with cICA lesions . |
|---|---|---|
| Abnormal TCD | 18 | 1 (6) |
| Conditional TCD | 13 | 1 (8) |
| Inadequate TCD | 11 | 0 (0) |
| Low-velocity/asymmetric TCD | 2 | 0 (0) |
| Acute stroke | 7 | 4 (57) |
| Previous stroke | 10 | 4 (40) |
| Other neurological symptoms* | 4 | 0 (0) |
| Pre-BM transplantation | 2 | 0 (0) |
| Total | 67 | 10 (15) |
Patients with stroke also had TCD abnormalities, but the primary indication for MR scan in these cases was for investigation of acute neurological event or monitoring progression of cerebral ischemia and cerebrovascular disease after a previous stroke.
Other neurological symptoms included severe headache (n = 2) and possible transient ischemic attack (n = 2).
Clinical and radiological features and details of treatment in patients with cervical ICA stenosis or occlusion
| Patient no. . | Age*/sex . | Clinical events . | Cranial MRI . | Cranial MRA . | Cervical MRA . | Management . |
|---|---|---|---|---|---|---|
| 1 | 16/M | Age 3: TIA (ataxia) Age 16: left hemiparesis Age 20: Recurrent TIAs | Age 3: Normal Age 16: Subsegmental right MCA infarct, DWS left ischemic lesions unchanged at age 20 | No evidence of intracranial vascular stenosis age 3, 16, and 20 | Occlusive lesion proximal right cICA, residual stump at origin of right cICA (Figure 1A), persisting occlusion age 20 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo, aspirin from age 20 |
| 2 | 13/M | Age 13: left hemiparesis, right headache, swallowing difficulties, absent gag Age 14: Recurrent AIS | Age 13 (after AIS): large acute infarct in right MCA territory | No evidence of intracranial vascular stenosis age 13 and 14 | Occlusive lesion proximal right cICA, tapering narrowing of residual stump, persisting occlusion at age 14 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo |
| 3 | 4/F | Age 4: TIA followed by left hemiparesis, left VII, left XII 2 mo later | Extensive left cortical and DWS infarct unchanged at age 5 and age 7 | Minor irregularity of left MCA Normal right-sided vessels | Occlusive lesion proximal left cICA, tapering narrowing of residual stump, persisting occlusion at age 5 and 7 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo |
| 4 | 4/F | Age 4: right hemiparesis, right VII 2 weeks after Varicella, URTI, bilateral cervical lymphadenopathy (recurrent AIS 3 mo) | Extensive left cortical and DWS infarct in left MCA and ACA territories, area-restricted diffusion within previously infarcted area on follow-up scan with recurrent AIS | Attenuated left MCA but not focally stenotic, mild stenosis of right ACA | Severe stenotic lesion proximal left cICA, flame-shaped proximal vessel, and distal string sign (Figure 2), thrombus within cICA at presentation with AIS, partial recanalization of vessel on follow-up age 5 and 6 | Acute exchange transfusion followed by chronic transfusion program |
| 5 | 5/M | Age 2: Probable AIS (acute neurological event in Africa) | Mature infarct left ACA/MCA watershed distribution | No intracranial vasculopathy | Severe stenotic lesion in distal left cICA Tortuous vertebral arteries and prominent tortuous anterior spinal artery (Figure 3) | Chronic transfusion program |
| 6 | 20/M | Age 2 and age 7 transient right hemiplegia, behavioral problems, deteriorating IQ | Age 7-15: annual or biannual scans, bilateral DWS infarcts and left caudate. Age 20: extensive infarction in left DWS and cortex, and right-sided infarction in DWS and focally in occipital cortex | Turbulent flow cranial ICA bilaterally from age 7 to 15, no apparent cerebrovascular disease age 20 | Severe stenotic lesion proximal left cICA, flame-shaped proximal vessel, and distal string sign (Figure 1B) | Chronic transfusion program age 7-14, left-sided EDAS procedure at age 11 Hydroxyurea and venesections at age 15-20 Aspirin and hydroxyurea from age 20 |
| 7 | 34/F | Age 22: Seizure, collapse Age 34: TIA | Age 22 (after AIS): Focal infarct right parietal cortex Age 27: Bilateral cortical ischemia worse on right side, unchanged at age 34 | No stenotic lesions in intracranial vessels | Severe stenotic lesion proximal right cICA, flame-shaped proximal vessel, and distal string sign (Figure 1C) | Regular exchange transfusions at age 22 Aspirin added at age 34 |
| 8 | 12/M | Age 11: left hemiparesis, left VII, left XII | Age 12: right frontal cortical infarct, small lesion in left DWS | Moderate right terminal ICA stenosis | Occlusion of proximal right cICA, flame-shaped appearance of residual stump | Chronic transfusion program at age 11 |
| 9 | 14/F | None | No ischemic change age 10, 14, and 16 | Normal intracranial vessels at age 10, 14, and 16 | Severe stenotic lesion proximal right cICA, attenuation of distal vessel (Figure 1D), uchanged at age 16 | Chronic top-up transfusion program at age 14 |
| 10 | 8/M | None | Right DWS ischemia | Normal intracranial vessels | Moderate stenotic lesion in distal right cICA (Figure 1E) | Regular top-up transfusion program at age 8 |
| Patient no. . | Age*/sex . | Clinical events . | Cranial MRI . | Cranial MRA . | Cervical MRA . | Management . |
|---|---|---|---|---|---|---|
| 1 | 16/M | Age 3: TIA (ataxia) Age 16: left hemiparesis Age 20: Recurrent TIAs | Age 3: Normal Age 16: Subsegmental right MCA infarct, DWS left ischemic lesions unchanged at age 20 | No evidence of intracranial vascular stenosis age 3, 16, and 20 | Occlusive lesion proximal right cICA, residual stump at origin of right cICA (Figure 1A), persisting occlusion age 20 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo, aspirin from age 20 |
| 2 | 13/M | Age 13: left hemiparesis, right headache, swallowing difficulties, absent gag Age 14: Recurrent AIS | Age 13 (after AIS): large acute infarct in right MCA territory | No evidence of intracranial vascular stenosis age 13 and 14 | Occlusive lesion proximal right cICA, tapering narrowing of residual stump, persisting occlusion at age 14 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo |
| 3 | 4/F | Age 4: TIA followed by left hemiparesis, left VII, left XII 2 mo later | Extensive left cortical and DWS infarct unchanged at age 5 and age 7 | Minor irregularity of left MCA Normal right-sided vessels | Occlusive lesion proximal left cICA, tapering narrowing of residual stump, persisting occlusion at age 5 and 7 | Acute exchange transfusion followed by chronic transfusion program, anticoagulation for 3 mo |
| 4 | 4/F | Age 4: right hemiparesis, right VII 2 weeks after Varicella, URTI, bilateral cervical lymphadenopathy (recurrent AIS 3 mo) | Extensive left cortical and DWS infarct in left MCA and ACA territories, area-restricted diffusion within previously infarcted area on follow-up scan with recurrent AIS | Attenuated left MCA but not focally stenotic, mild stenosis of right ACA | Severe stenotic lesion proximal left cICA, flame-shaped proximal vessel, and distal string sign (Figure 2), thrombus within cICA at presentation with AIS, partial recanalization of vessel on follow-up age 5 and 6 | Acute exchange transfusion followed by chronic transfusion program |
| 5 | 5/M | Age 2: Probable AIS (acute neurological event in Africa) | Mature infarct left ACA/MCA watershed distribution | No intracranial vasculopathy | Severe stenotic lesion in distal left cICA Tortuous vertebral arteries and prominent tortuous anterior spinal artery (Figure 3) | Chronic transfusion program |
| 6 | 20/M | Age 2 and age 7 transient right hemiplegia, behavioral problems, deteriorating IQ | Age 7-15: annual or biannual scans, bilateral DWS infarcts and left caudate. Age 20: extensive infarction in left DWS and cortex, and right-sided infarction in DWS and focally in occipital cortex | Turbulent flow cranial ICA bilaterally from age 7 to 15, no apparent cerebrovascular disease age 20 | Severe stenotic lesion proximal left cICA, flame-shaped proximal vessel, and distal string sign (Figure 1B) | Chronic transfusion program age 7-14, left-sided EDAS procedure at age 11 Hydroxyurea and venesections at age 15-20 Aspirin and hydroxyurea from age 20 |
| 7 | 34/F | Age 22: Seizure, collapse Age 34: TIA | Age 22 (after AIS): Focal infarct right parietal cortex Age 27: Bilateral cortical ischemia worse on right side, unchanged at age 34 | No stenotic lesions in intracranial vessels | Severe stenotic lesion proximal right cICA, flame-shaped proximal vessel, and distal string sign (Figure 1C) | Regular exchange transfusions at age 22 Aspirin added at age 34 |
| 8 | 12/M | Age 11: left hemiparesis, left VII, left XII | Age 12: right frontal cortical infarct, small lesion in left DWS | Moderate right terminal ICA stenosis | Occlusion of proximal right cICA, flame-shaped appearance of residual stump | Chronic transfusion program at age 11 |
| 9 | 14/F | None | No ischemic change age 10, 14, and 16 | Normal intracranial vessels at age 10, 14, and 16 | Severe stenotic lesion proximal right cICA, attenuation of distal vessel (Figure 1D), uchanged at age 16 | Chronic top-up transfusion program at age 14 |
| 10 | 8/M | None | Right DWS ischemia | Normal intracranial vessels | Moderate stenotic lesion in distal right cICA (Figure 1E) | Regular top-up transfusion program at age 8 |
TIA indicates transient ischemic attack; VII, 7th nerve palsy; XII, 12th nerve palsy; DWS, deep watershed; and EC-IC, extracranial-intracranial.
Age is at the time of cICA lesion diagnosis.
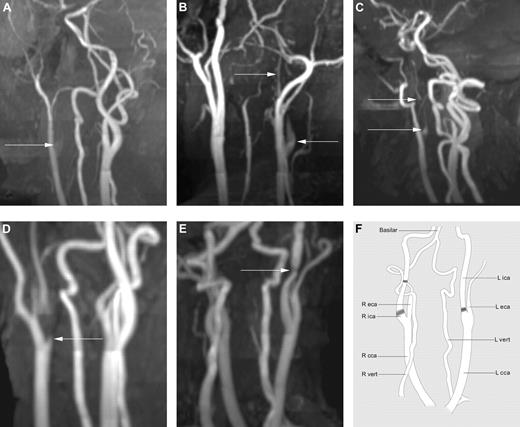
Maximum-intensity projection reconstructions in varying obliquities of 3D time-of-flight MRA of the neck. (A) Patient 1 demonstrates a flame-shaped origin to the ICA (arrow) that is completely occluded without any distal flow. (B) Patient 6 demonstrates a flame-shaped narrowing (arrow) to the origin of the right ICA that is of a much-reduced caliber (string sign) distally (arrow). (C) Patient 7 demonstrates a much-reduced caliber ICA (arrow) from its origin, the so-called “string” sign. (D) Patient 9 demonstrates a focal stenosis at the origin of the ICA (arrow) with reduced caliber distal to the stenosis. (E) Patient 10 demonstrates a short focal stenosis (arrow) well above the origin of the ICA that is otherwise of normal caliber. (F) Line drawing of unrotated cMRA illustrating the normal anatomy of arterial vessels in the neck and indicating the sites of stenosis and occlusion in patients 1-10 (shaded). eca indicates external carotid artery; cca, common carotid artery; and vert, vertebral artery.
Maximum-intensity projection reconstructions in varying obliquities of 3D time-of-flight MRA of the neck. (A) Patient 1 demonstrates a flame-shaped origin to the ICA (arrow) that is completely occluded without any distal flow. (B) Patient 6 demonstrates a flame-shaped narrowing (arrow) to the origin of the right ICA that is of a much-reduced caliber (string sign) distally (arrow). (C) Patient 7 demonstrates a much-reduced caliber ICA (arrow) from its origin, the so-called “string” sign. (D) Patient 9 demonstrates a focal stenosis at the origin of the ICA (arrow) with reduced caliber distal to the stenosis. (E) Patient 10 demonstrates a short focal stenosis (arrow) well above the origin of the ICA that is otherwise of normal caliber. (F) Line drawing of unrotated cMRA illustrating the normal anatomy of arterial vessels in the neck and indicating the sites of stenosis and occlusion in patients 1-10 (shaded). eca indicates external carotid artery; cca, common carotid artery; and vert, vertebral artery.
Imaging of patient 4. (A) Maximum-intensity projection of a 3D time-of-flight MRA of the neck vessels in an oblique projection. Flow is just visible at the origin of the left ICA (arrow), but distal flow is attenuated and discontinuous. (B) Maximum-intensity projection of the repeat 3D time-of-flight MRA of the neck vessels at 3 months. The left ICA now shows continuous flow, but remains of diffusely reduced caliber (arrows). The linear artifact across all the vessels is due to the multi-slab MR technique. (C) Axial T1-weighted image with fat saturation showing high signal thrombus in the lumen of the left ICA (arrow), whereas no hematoma is seen in the wall of the vessel. (D) Line drawing illustrating anatomical features of panel C. ijv indicates internal jugular vein; and vert, vertebral artery.
Imaging of patient 4. (A) Maximum-intensity projection of a 3D time-of-flight MRA of the neck vessels in an oblique projection. Flow is just visible at the origin of the left ICA (arrow), but distal flow is attenuated and discontinuous. (B) Maximum-intensity projection of the repeat 3D time-of-flight MRA of the neck vessels at 3 months. The left ICA now shows continuous flow, but remains of diffusely reduced caliber (arrows). The linear artifact across all the vessels is due to the multi-slab MR technique. (C) Axial T1-weighted image with fat saturation showing high signal thrombus in the lumen of the left ICA (arrow), whereas no hematoma is seen in the wall of the vessel. (D) Line drawing illustrating anatomical features of panel C. ijv indicates internal jugular vein; and vert, vertebral artery.
Imaging of patient 5. (A) Severe stenosis in the distal cICA can be seen, along with tortuosity of the vertebral arteries and a prominent, tortuous anterior spinal artery. (B) Line drawing illustrating anatomical features. (C) Audible turbulence in terminal ICA on TCD sonogram (vertical arrow).
Imaging of patient 5. (A) Severe stenosis in the distal cICA can be seen, along with tortuosity of the vertebral arteries and a prominent, tortuous anterior spinal artery. (B) Line drawing illustrating anatomical features. (C) Audible turbulence in terminal ICA on TCD sonogram (vertical arrow).
Radiologic features of cICA stenosis or occlusion
The majority of the lesions were in the proximal cICA ∼ 1 cm from its origin. In patients 5 and 10, the lesion was in the distal cICA at the C2/3 level (Figures 1E and 3A). There was no significant predilection for right or left side. The vessel was completely occluded in patients 1, 2, 3, and 8; the remainder had severe stenotic lesions. Radiologic features consistent with dissection include flame-shaped or tapered narrowing (patients 1, 2, 3, 4, 6, 7, and 8) and string sign (patients 4, 6, and 7). An axial T1 fat-saturation sequence was only performed in one patient (patient 4) and did not demonstrate a mural hematoma; instead, hematoma was identified in the vessel lumen (Figure 2C). Axial MRA was negative for vessel wall thickening in all patients. The cICA lesions were not due to extension of an intracranial occlusion—patients 3 and 4 had mild irregularity of the ipsilateral middle cerebral artery (MCA) and patient 8 had mild ipsilateral terminal ICA attenuation, but these abnormalities were not contiguous with the cICA lesions. The remaining patients all had patent intracranial vessels.
To further study the cICA lesions, cervical arterial Doppler scanning was done in patients 6, 7, 9, and 10. High-grade stenoses were demonstrated in patients 7, 9, and 10 at sites corresponding to lesions seen on cMRA. In patient 6, carotid Doppler flow was apparently normal, but there was turbulent flow consistent with a stenosis in the subcavernous portion of the ICA. cMRA in this patient showed severe left-sided cICA stenosis with flame-shaped narrowing and string sign together with a prominent external carotid artery. Possible explanations for the apparently normal carotid Doppler traces include inadvertent scanning of the external carotid artery and intermittent resumption of flow because a nonvisualized aneurysmal flap no longer occluded the vessel.
Cerebral ischemic lesions on MRI are described in Table 2. Lesions ipsilateral to the cICA stenosis were seen in patients 1 through 8, all of whom had suffered AIS. Patient 10 had a typical pattern of silent cerebral ischemia in an MCA/anterior cerebral artery (ACA) watershed distribution affecting the deep white matter and sparing the cortex ipsilateral to the cICA lesion. Patient 9 had no evidence of cerebral infarction despite a tight stenosis at the origin of the right ICA.
Clinical features and evolution of cICA lesions
Most of the patients had been followed from birth or early childhood, and some also had serial TCD and MRI up to the time of the acute neurologic event or identification of cICA lesion. Clinical features are summarized in Table 2.
In patient 1, the presence of a lesion long before the AIS is suggested by the diagnosis of a TIA, and the concurrent finding of low and asymmetric TCD velocities on the right side at the age of 3. This patient went on to have AIS in the right MCA territory and occlusion of the right cICA at the age of 16. He also had retinal artery occlusion on the side of the lesion, presenting with uniocular partial visual loss concurrently with AIS. These ischemic events are both likely to be embolic in origin.
Patient 2 had cervical lymphadenopathy due to Hodgkin disease, and AIS occurred after a surgical procedure on the same side of the neck for insertion of a PORT-A-CATH. The origin of the cICA occlusion was distant to the affected lymphoid tissue and there was no evidence of trauma to, or hematoma surrounding, the ICA. We suggest that occlusion of the cICA lesion occurred during neck manipulation under general anesthesia for line insertion, perhaps causing dissection of an already diseased vessel wall.
In patient 4, high stroke risk and the possibility of a left cICA stenosis was evident in hindsight on the basis of asymmetric TCD velocities that were abnormal on the opposite side of the cICA lesion. This patient suffered AIS 4 weeks later in the left MCA and ACA vascular territories before repeat TCD could be done and transfusions started, and was found to have a thrombotic occlusion of the left cICA (Figure 2C). She suffered a recurrent AIS 3 months later in the same vascular territory despite transfusion to a hemoglobin S percentage of 30%. This patient was not anticoagulated, and we suggest that this AIS was due to thrombotic embolus from the cICA. This patient's case is also interesting because it shows that an occluded vessel can partially recanalize, giving an MRA the appearance of a “string sign” (Figure 2B).
In patients 5, 7, and 8, we suggest that the severe cICA lesions were responsible for previous AIS. These strokes were in the affected vascular territory, and there was minimal intracerebral vascular disease to account for the ischemic damage. Patients 7 and 8 had cortical infarcts in branch MCA vascular territory that were associated with radiographic features of ipsilateral cICA dissection, and we suggest that these patients suffered thromboembolic strokes with thrombotic narrowing or occlusion of the cICA.
In patient 6, the association between the cICA lesion and cerebral ischemia is less clear. He had a transient right hemiparesis at ages 2 and 6 and was investigated for behavioral problems, deteriorating cognitive function, and severe headaches from mid-childhood. Serial TCD velocities evolved from normal (at age 4) to high conditional (at age 7; 198 cm/s right MCA, 178 cm/s left MCA). By age 11, there was no TCD flow detectable on the left side. This patient had serial MR imaging from age 7, which showed bilateral deep white matter ischemia and left caudate infarct. MRA showed turbulent flow bilaterally in the terminal ICAs. At age 11, he had digital subtraction angiography from a right femoral approach with catheterization of both internal and external carotid and the left vertebral arteries. This showed diffuse and focal arterial narrowing that was most conspicuous in the distal ACAs, with leptomeningeal collaterals and no occlusion of the neck vessels. Because there was reduced flow in the left frontal region on single photon emission computed tomography, this patient subsequently underwent a left-sided encephaloduroarteriosynangiosis (EDAS) procedure at 11 years of age, which was uneventful, with significant reduction in headache. cMRA was done for the first time at age 20, showing flame-shaped occlusion of the left cICA (Figure 1B). In this patient, it is unclear when the cICA lesion occurred and how it evolved.
Patients 9 and 10 had localized stenotic, nonocclusive cICA lesions that were identified with cMRA scanning for investigation of high TCD velocities. Patient 9 had no evidence of cerebral infarction, whereas patient 10 had multiple silent cerebral infarcts on the side ipsilateral to the cICA stenosis. The ischemic lesions were distributed in the deep white matter in the vascular watershed area of the MCA and ACA rather than involving the cortex in the vascular territory of either of these vessels, which is compatible with hemodynamic hypoperfusion rather than an embolic event. These 2 patients are likely to represent the stage in evolution of a cICA lesion before occlusion and AIS. Both patients have been transfused regularly since identifying the lesion and have not yet suffered AIS.
TCD findings in patients with cICA stenosis/occlusion
Cervical arterial lesions were associated with altered flow on TCD (Table 3). Turbulent flow related to a stenosis in the subcavernous portion of the cICA was demonstrable in patient 5 (Figure 3C). In patients 1, 2, 3, and 4, there was no flow detectable in the terminal ICA and proximal ACA and MCA ipsilateral to the cICA lesion after AIS. In patients 1 and 4 before AIS; patients 4, 6, and 8 after AIS; and in patients 9 and 10 with stenotic cICA lesions and no history of AIS, velocities on the side ipsilateral to the lesion were normal or low. On the contralateral side, velocities were either conditional or abnormal. These high velocities were found in the terminal ICA, MCA, or ACA, suggesting a general compensation for low flow on the affected side by increasing the flow to the unaffected side, which might assist in perfusion of both cerebral hemispheres via the Circle of Willis.
TCD time averaged maximum flow velocity (Vmax) before and after AIS in patients with cICA stenosis or occlusion
| Patient no. . | Age at AIS . | Side of lesion . | Vmax, cm/s . | |||
|---|---|---|---|---|---|---|
| Pre-AIS . | Post-AIS . | |||||
| Left . | Right . | Left . | Right . | |||
| 1 | 16 | R | 82 MCA | 32 MCA | NS | NS |
| 2 | 13 | R | ND | ND | ND | ND |
| 3 | 4 | L | ND | ND | NS | 104 MCA |
| 4 | 4 | L | 158 MCA | 201 MCA | NS | 225 ACA |
| 5 | 2 | L | ND | ND | 100 tICA | 116 tICA |
| 6 | 7 | L | 174 MCA | 198 MCA | NS | 120 MCA |
| 7 | 22 | R | ND | ND | 98 tICA | 83 MCA |
| 8 | 12 | R | ND | ND | 201 MCA | 157 tICA |
| 9 | NA | R | 232 MCA | 157 tICA | NA | NA |
| 10 | NA | R | 189 tICA | 150 tICA | NA | NA |
| Patient no. . | Age at AIS . | Side of lesion . | Vmax, cm/s . | |||
|---|---|---|---|---|---|---|
| Pre-AIS . | Post-AIS . | |||||
| Left . | Right . | Left . | Right . | |||
| 1 | 16 | R | 82 MCA | 32 MCA | NS | NS |
| 2 | 13 | R | ND | ND | ND | ND |
| 3 | 4 | L | ND | ND | NS | 104 MCA |
| 4 | 4 | L | 158 MCA | 201 MCA | NS | 225 ACA |
| 5 | 2 | L | ND | ND | 100 tICA | 116 tICA |
| 6 | 7 | L | 174 MCA | 198 MCA | NS | 120 MCA |
| 7 | 22 | R | ND | ND | 98 tICA | 83 MCA |
| 8 | 12 | R | ND | ND | 201 MCA | 157 tICA |
| 9 | NA | R | 232 MCA | 157 tICA | NA | NA |
| 10 | NA | R | 189 tICA | 150 tICA | NA | NA |
ND indicates not done; NA, not applicable; NS, no signal obtainable; and tICA, terminal ICA.
Management
Patients 1-3 were anticoagulated acutely after presenting with AIS, initially with low molecular weight heparin and then with Coumadin. Coumadin was continued for 3 months and was dose adjusted to maintain the international normalized ratio in the range of 2 to 3. None of the patients received aspirin acutely, although patients 1, 6, and 7 are currently being treated with aspirin due to persistent transient ischemic attacks several years after AIS despite regular transfusion. All of the patients with acute stroke also underwent immediate exchange transfusion to bring the hemoglobin saturation to below 30%, and were maintained thereafter on a long-term transfusion program.
Discussion
We have described the clinical and radiologic features of cICA occlusion or stenosis in SCD. Two recent studies have reported cICA lesions in SCD using cervical Doppler. Gorman et al identified 4 of 131 children with cICA lesions, 3 of whom had a stroke.11 In another Doppler study, Deane et al identified 13 of 236 children with cICA stenosis or occlusion, and showed that children with these lesions had a 36-fold increased risk of stroke.12 In both of these studies, the investigators recommended that routine cervical carotid Doppler screening should be offered in addition to TCD to children with SCD. In another study, Calviere et al reported the case of a 19-year-old patient presenting with acute stroke due to cICA occlusion. They subsequently scanned 15 children with carotid Doppler, but did not find any others with stenotic lesions.6
We are aware of only one other report of a series of patients with SCD undergoing cMRA.14 In that study, the purpose was to compare Doppler velocities in the MCA, terminal ICA, and extracranial ICA in children unaffected with vascular disease, so children with a previous history of stroke or cerebrovascular lesions on MRA were excluded. Our study is different, representing the other end of the vasculopathy spectrum: patients with a history of neurologic events or likely to have vascular lesions on the basis of TCD results.
cMRA has widely replaced catheter angiography in the diagnosis of carotid artery stenosis and dissection in the non-SCD population.13 A large series using MRI and MRA reported 24 dissections on MRA in 180 patients. For carotid artery dissections, MRI and MRA had a sensitivity of 95% and a specificity of 99%.15 Vessel tortuosity can affect the MRA signal because turbulent flow can cause intravoxel dephasing of the signal and produce signal dropout mimicking a stenosis. However, all but one of the stenoses described in our series were not at sites of vessel tortuosity, as assessed by following the vessel orientation on sequential axial MRA images. In 1 patient (patient 10), there was a moderate stenosis at a site of tortuosity; however, there was a similar tortuosity on the contralateral ICA at the same location, which did not produce the appearance of a stenosis, indicating that there was more than tortuosity causing the signal loss.
The study provides some insights into the pathogenesis and evolution of cICA lesions. It is possible that the underlying pathology is similar to the large vessel damage seen in intracerebral vessels. The young age at which several of our patients presented with AIS or TIA implies that the extracerebral vascular disease in the ICA occurs at the same age as intracerebral disease. It is not clear whether the subsequent pathologic course involves thrombotic occlusion of a narrowed vessel or occlusion related to dissection of the vessel wall. The clinical and radiologic features of acute dissection include acute stroke with facial or neck pain, lower cranial nerve abnormalities, and Horner syndrome. Radiologic findings include flame-shaped dilatation, tapering narrowing, string sign, vessel wall enlargement, and thrombus visible within the vessel wall.16 Our 10 patients had some of these clinical and radiologic features, and in 7, the diagnosis was considered to be dissection. In the post mortem series by Rothman et al,2 patients with cerebral infarction had organized mural thrombus alone or in combination with recanalized transluminal thrombi predominantly in the supraclinoid internal carotid and proximal MCA and ACA. The thrombi were often associated with reduplication of the internal elastic membrane and medial scarring. One patient had cICA occlusion, and the histological changes were the same as those observed in intracerebral vessels. Dissection of the cICA in non-SCD patients seems to involve hemorrhage and thrombus within the vessel wall, perhaps related to excessive stretching or rotation of the neck, and occurs where the vessel is most mobile. Intraluminal thrombus may then form where the overlying endothelium is damaged.16 In SCD, this is probably the result of chronic adhesion and shearing of abnormal sickle hemoglobin–containing RBCs, leading to pathologic thickening of the cICA wall likely to predispose to dissection. In the present study, dissection of a previously damaged extracranial vessel appears to occur in SCD, but more data are required in patients with acute stroke to determine its relative importance compared with intracranial vasculopathy and whether intracranial dissection occurs.
The TCD findings in these patients were also noteworthy. There was significant asymmetry between the flow velocities, and often remarkably high values on the side contralateral to the cICA stenosis/occlusion. This is presumably because of compensatory hyperemia and has not previously been described in TCD studies (although high contralateral velocity on cervical carotid Doppler has been noted11 ). We think it is important that ultrasonographers and clinicians interpreting TCD scans in SCD children are aware that high flow velocities are not always related to ipsilateral stenotic lesions.
The decision to anticoagulate patients with AIS associated with cICA lesions was based on recommendations for adults and children with acute carotid dissection, recognizing that these guidelines are based on anecdotal evidence and case series, rather than on randomized controlled trials.16,17 In the case of SCD, anticoagulation is relatively contraindicated because of the risk of cerebral hemorrhage associated with cerebral vasculopathy, and we are not aware of any published data on outcomes in children or adults with AIS treated with anticoagulants. We made the decision to anticoagulate patients 1-3 on clinical and radiologic evidence of possible or probable carotid artery dissection. We carefully examined acute MRI and MRA images and were satisfied that none of the patients had evidence of cerebral hemorrhage or moyamoya formation. None of these patients had recurrence of AIS, although the vessel has remained occluded on follow-up scans in all 3. Patient 4 was not anticoagulated because the attending clinician was concerned about the risk of hemorrhage. This patient had demonstrable thrombosis within the vessel lumen and had recurrent AIS 3 months later, which may have been due to a thromboembolic event; we think that this might have been prevented had she been anticoagulated acutely. Based on our experience, anticoagulation appears to be safe and may be beneficial in patients presenting with AIS and cICA lesions provided there is no evidence of intracranial hemorrhage or moyamoya.
Regular transfusion may be indicated for those with high TCD velocities, even if the cICA lesion is contralateral to the high velocity, but it is not certain that there will be a beneficial effect in primary prevention of AIS related to cICA occlusion similar to intracerebral vascular occlusion.9 Two patients in our series with high TCD velocities have been regularly transfused for primary stroke prevention, and at present both are stroke-free.
Patients with cICA lesions may benefit from surgical revascularization, but there are scant data in SCD. Possible approaches include carotid endarterectomy and EDAS. The former is often done to relieve stenotic lesions and to prevent stroke in patients with atherosclerotic disease, and we have considered it for one of our patients (patient 9); however, the risk of thromboembolic AIS may be high if this procedure is attempted in a young patient with SCD. EDAS has been recommended in patients with SCD and moyamoya vessels, but its efficacy is not yet established.18 There are no previous reports of EDAS in patients with cICA lesions. Patient 6 had EDAS 9 years before the cICA lesion was identified, and it is possible that the lesion may have been provoked by direct carotid angiography or by the EDAS procedure itself. It is important to be aware that these patients may be at risk of dissection or occlusion during general anesthesia if the neck is vigorously manipulated. We think this is a likely explanation for the AIS in patient 2.
This is not a clinical trial and the conclusions should be interpreted cautiously. Although prospective in the sense that all consecutive patients who met the study criteria underwent cervical MRA, it was not possible to include neurologically normal controls with standard-risk TCD scans because they would not have met the clinical criteria for MRI scanning in our program. The prevalence of cICA lesions in the general SCD population is therefore unclear.
In conclusion, routine cMRA scanning of patients with previous or suspected cerebrovascular disease has shown an unexpectedly high proportion of patients with cICA lesions, which have hitherto been underdiagnosed because the neck vessels are not routinely studied. These lesions are associated with AIS, possibly because of thrombotic occlusion of the cICA and distal thromboembolism. It is not clear whether dissection of the vessel wall initiates the acute event, but most of our patients had some of the typical radiologic features. We suggest that anticoagulation is important after AIS in this setting to prevent thrombotic extension and thromboembolic stroke, and our experience indicates that it can be given safely without provoking hemorrhagic complications. There are no specific diagnostic clinical features of AIS due to cICA occlusion, and we recommend that all patients with SCD and acute AIS have cMRAs including axial T1 fat-saturation sequence to identify thrombotic occlusion or dissection that may justify anticoagulation. For children with Doppler abnormalities (high-risk, conditional, low-velocity, asymmetric, or absent trace), we recommend scanning of the neck vessels (carotid Doppler and/or MRA). When TCD velocities are abnormal, as we have observed in the vessels contralateral to the cICA lesion, it is reasonable to offer regular transfusion. Other options include hydroxyurea, antiplatelet agents, and revascularization surgery, which should be evaluated in a clinical trial setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nelly Pecheva and Bryony Cohen, Department of Medical Illustration, St Bartholomew's Hospital, for preparing the illustrations.
Authorship
Contribution: P.T.T. contributed patients, performed the majority of the TCD scans, analyzed the data, and wrote the manuscript; J.E. performed all of the MRI scans, contributed to writing the manuscript, and prepared the images; P.B. reviewed the MRI scans and contributed to writing the manuscript; C.H., C.A., N.G., S.W., and B.K. contributed patients and to writing and reviewing the manuscript; and F.J.K. followed some of the patients, performed some of the TCD scans, analyzed the data, and contributed to writing and reviewing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Paul T. Telfer, Department of Pediatric Haematology and Oncology, Pathology and Pharmacy Building, Royal London Hospital, 80 Newark Street, London E1 2ES, United Kingdom; e-mail: paul.telfer@bartsandthelondon.nhs.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal