Abstract
The mechanisms by which innate immune signals regulate alloimmune responses remain poorly understood. In the present study, we show by intravital 2-photon microscopy direct interactions between graft-infiltrating neutrophils and donor CD11c+ dendritic cells (DCs) within orthotopic lung allografts immediately after reperfusion. Neutrophils isolated from the airways of lung transplantation recipients stimulate donor DCs in a contact-dependent fashion to augment their production of IL-12 and expand alloantigen-specific IFN-γ+ T cells. DC IL-12 expression is largely regulated by degranulation and induced by TNF-α associated with the neutrophil plasma membrane. Extended cold ischemic graft storage enhances G-CSF–mediated granulopoiesis and neutrophil graft infiltration, resulting in exacerbation of ischemia-reperfusion injury after lung transplantation. Ischemia reperfusion injury prevents immunosuppression-mediated acceptance of mouse lung allografts unless G-CSF–mediated granulopoiesis is inhibited. Our findings identify granulopoiesis-mediated augmentation of alloimmunity as a novel link between innate and adaptive immune responses after organ transplantation.
Introduction
Ischemia reperfusion injury is a frequent complication after lung transplantation and is considered to be the principal mechanism leading to primary graft dysfunction, a form of acute pulmonary injury characterized by impaired oxygen exchange and the appearance of pulmonary infiltrates. In addition to its acute effects, primary graft dysfunction is also associated with an increased risk for the development of chronic rejection and late mortality.1 Strikingly, there has been little change in the frequency of primary graft dysfunction in the last 3 decades, which still occurs in up to 80% of lung transplantation recipients.2 Neutrophils are critical mediators of ischemia reperfusion injury after lung transplantation.3 Recent work from our laboratory has shown that syngeneic lung transplantation stimulates the expansion of BM-resident neutrophil progenitors, which leads to the accumulation of neutrophils within the peripheral blood and graft tissues.4 This stress-related or “emergency” response has also been characterized during infection and can be driven by several granulopoietic cytokines, including G-CSF, GM-CSF, and IL-3.5 G-CSF in particular has been shown to accumulate in the peripheral blood after lung transplantation and to promote acute pulmonary injury, but its impact on alloimmunity remains unclear.4,6
It has been the prevailing view that neutrophils stimulate adaptive immune responses through their function as phagocytes by killing and engulfing pathogens in peripheral tissues and then delivering microbial antigens to dendritic cells (DCs) in the draining lymph nodes. In addition, it is recognized that both neutrophils and APCs are recruited to inflammatory foci, suggesting the possibility of regulatory interactions between these 2 populations outside of secondary lymphoid organs. Ex vivo studies have indicated that neutrophils may stimulate the maturation of DCs through direct contact mediated by CD11b and DC-SIGN.7 However, it remains to be determined whether neutrophil-DC interactions occur in vivo within nonlymphoid tissues.
By using intravital 2-photon (2P) microscopy, we recently observed in a mouse model of orthotopic lung transplantation that large numbers of neutrophils enter the graft tissue where many donor-derived DCs reside, raising the possibility of functionally significant neutrophil-DC interactions. In the present study, we report that neutrophils interact with DCs for extended periods of time within mouse orthotopic lung allografts. Extended cold preservation time of lungs leads to ischemia reperfusion injury that drives G-CSF–dependent granulopoiesis and accumulation of neutrophils within pulmonary tissues, leading to IL-12 production by DCs, enhanced Th1 alloimmunity, and acute rejection. Our results reveal a previously unrecognized link between innate and adaptive immune responses after lung transplantation, and could have important implications for a wide variety of immune-mediated pulmonary diseases.
Methods
Mice
Lung transplantation, injury assessment, and recipient treatment
Left Balb/c or C57BL/6 (B6) lungs were stored under cold ischemia (CI) conditions for 1 hour (Min CI) or 18 hours (Ext CI) in a low-potassium dextran glucose solution at 4°C before transplantation into B6 recipients.10,11 Recipients were treated with MR1 (250 μg on day 0) and CTLA4-Ig (200 μg on day 2; Bio-X-Cell).12 Neutrophils were depleted through IV injection of Ly6G-specific Abs (1A8; 250 μg; Bio-X-Cell) 4 hours before surgery, as described previously.4 G-CSF was neutralized through IV injection of 200 μg of G-CSF–specific Abs (Peprotech) 1 hour before surgery. Ten micrograms of mouse recombinant G-CSF (Peprotech) was injected intravenously immediately after reperfusion. IL-12 was neutralized with 500 μg of IL-12–neutralizing Abs (C17.8; Bio-X-Cell) administered by IP injection on postoperative day (POD) 0 and 3. Evans blue dye and arterial blood gases were measured as described previously.4
2P microscopy
After 18 hours of cold storage, B10.BR CD11c-EYFP lungs were transplanted into B6 LysM-GFP mice. Neutrophils are brightly labeled with green fluorescent protein (GFP) compared with macrophages and monocytes.8,13 Time-lapse imaging was performed 1 hour after reperfusion with a custom-built 2P microscope running ImageWarp Version 2.1 acquisition software (A&B Software).8 To visualize blood vessels, 15 μL of 655-nm nontargeted Q-dots suspended in 50 μL of PBS were injected intravenously before imaging. For time-lapse imaging of neutrophil-CD11c+ DC interactions in lung tissue, we averaged 15 video-rate frames (0.5 seconds per slice) during the acquisition to match the ventilator rate and to minimize movement artifacts. Each plane represents an image of 220 × 240 μm in the x and y dimensions. Twenty-one sequential planes were acquired in the z dimension (2.5 μm each) to form a z stack. Neutrophil contact time with DCs was measured using Imaris software (Bitplane).
Methylcellulose colony assay
In accordance with the manufacturer's recommendations, BM cells were suspended in Iscove MDM (StemCell Technologies) with 2% FBS and mixed with MethoCult M3234 methylcellulose medium (StemCell Technologies) supplemented with 10 ng/mL of mouse recombinant SCF, G-CSF, IL-3, and IL-6. 2.5 × 104 nucleated cells were plated in 2.5 mL of medium per dish. A CFU was defined as a colony with at least 30 cells. CFUs were counted and differentiated by morphology on day 7 with an inverted microscope at a 25× magnification. Colony size was estimated by dividing the total number of cells per plate by CFU. Cultures were plated in triplicate and CFU was calculated as a mean ± SEM.
Neutrophil culture
5 × 105 neutrophils were cultured alone or added to BM-derived DCs (BMDCs) at a 3 to 1 ratio in RPMI 1640 medium (Invitrogen), 0.05mM β-mercaptoethanol with 10% FCS. Neutrophils were preincubated for 20 minutes at 25°C in vehicle (0.5mM CaCl2, 1mM MgCl2, 20mM HEPES, pH 7.4, and 0.1% BSA) alone or with various concentrations of genistein (Sigma-Aldrich). Cytochalasin B (Sigma-Aldrich) stimulation was conducted in vehicle for 5 minutes at 25°C. When both agents were used, cytochalasin B stimulation followed immediately after genistein treatment. VAS2870 (Enzo Life Sciences) was solubilized in DMSO at 10 mg/mL and added to neutrophils suspended in vehicle at a final concentration of 10μM for 10 minutes at 25°C. All inhibitors and stimulators were removed from neutrophils by 2 washes with PBS before culture.
Neutrophil membrane extraction
5 × 106 neutrophils were fractioned for plasma membranes using the Qproteome Plasma Membrane Protein Kit (QIAGEN) in accordance with the manufacturer's recommendations. Plasma membrane fraction equivalents of 106 neutrophils were incubated with either TNF-α–neutralizing Abs or control Ig for 30 minutes at 4°C and then cocultured with 106 BMDCs for 24 hours before cytokine analysis.
Cytokine analysis
Lung tissue digests were examined as described previously.11 T cells were identified with fluorochrome-labeled Abs (eBioscience) specific for CD90.2 (30-H12), CD4 (RM4-5), and CD8 (53-6.7). T-cell intracellular expression of IFN-γ was measured as described previously.9 For intracellular IL-12 expression analysis, BMDCs or lung tissue digests were cultured for 4 hours at 37°C with 2μM monensin before staining with fluorochrome-labeled Abs specific for CD11c (N418), I-Ad (AMS-32.1; BD Biosciences), TNF-α (TN3-19.12), and IL-12 p40/70 (p40/p70). G-CSF, GM-CSF, and IL-3 concentrations in recipient serum were assessed with Quantikine ELISA kits (R&D Systems).4 IL-12p70, IL-6, IFN-γ, and TNF-α levels in the culture supernatants were measured with FlowCytomix kits (eBioscience) in accordance with the manufacturer's recommendations.
Neutrophil assessment and purification
Neutrophils were counted in the bronchoalveolar lavage (BAL) and peripheral blood with a HEMAVET analyzer (Drew Scientific). Neutrophils were purified by immunomagnetic bead-mediated negative selection with biotin-labeled Abs (eBioscience) specific for CD3e (145-2C11), CD4 (L3T4), CD8a (Ly-2), CD19 (eBio1D3), B220 (RA3-6B2), CD49b (DX5), CD11c (N418), I-A/I-E (M5/114.15.2), and CD115 (c-fms). Labeled cells were fractionated with streptavidin beads and LS MACS columns (Miltenyi Biotec). Immature BMDCs were prepared from T cell–depleted BM cells as described previously14 and cultured with 30 ng/mL of GM-CSF (Peprotech) and 30 ng/mL of IL-4 (Peprotech) for 7-10 days. BMDCs were then cultured with BAL neutrophils from lung recipients for an additional 24 hours before adoptive transfer or cytokine analysis.
Histology
Rejection was scored by a blinded pathologist (J.H.R.) and was based on criteria established by the International Society for Heart and Lung Transplantation for acute rejection: grade A0 = none, grade A1 = minimal, grade A2 = mild, grade A3 = moderate, and grade A4 = severe. Histology was analyzed with an Olympus BX51 microscope and all micrographs are represented at a 100× magnification using a 10× objective.
Statistics
Data were analyzed using Prism Version 5.0 software (GraphPad). An unpaired 2-tailed Student t test was used to evaluate pairs of means for significance. P < .05 was considered significant.
Results
Prolonged lung ischemia promotes emergency granulopoiesis
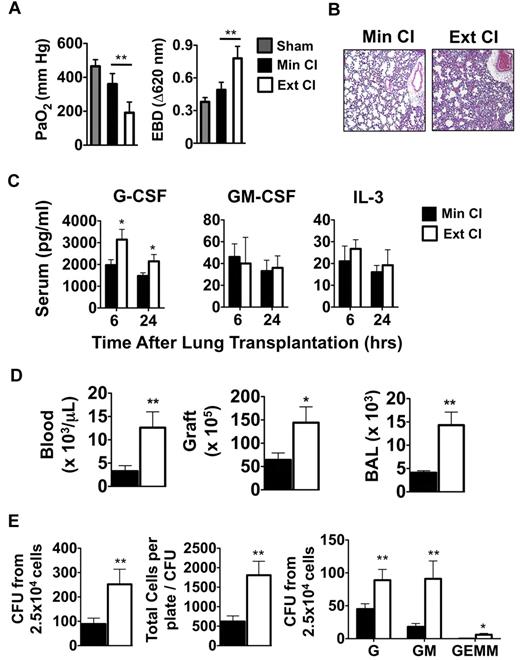
Based on clinical reports that prolonged graft ischemia adversely affects early graft function and long-term survival in lung transplantation recipients, we set out to examine the effects of extended periods of cold storage on pulmonary function in a mouse model of orthotopic vascularized pulmonary transplantation.15 We transplanted Balb/c lungs that had either undergone Min CI or Ext CI into immunosuppressed B6 mice and assessed graft function 24 hours later. Ext CI resulted in significantly worse acute graft injury, as evidenced by impaired oxygen exchange and pulmonary edema (Figure 1A-B) and was associated with significantly higher serum concentrations of G-CSF, but not other granulopoietic cytokines such as GM-CSF or IL-3 (Figure 1D). Consistent with severe acute graft injury in Ext CI lung recipients, we observed higher numbers of neutrophils within the pulmonary tissues and peripheral blood (Figure 1D). Neutrophil accumulation and acute graft injury in syngeneic lung recipients at 24 hours after engraftment is regulated by mechanisms that control the expansion of granulocyte progenitors within the BM.4 Accordingly, we investigated whether there was evidence of augmented BM emergency granulopoiesis in Ext CI lung allograft recipients. Compared with Min CI lung recipients, we observed significantly larger numbers of granulocyte progenitors and granulocyte progenitor colony sizes from the BM of Ext CI lung recipients, indicating that enhanced emergency granulopoiesis is correlated with the degree of acute lung allograft injury (Figure 1E).
Emergency granulopoiesis. (A) PaO2 (left) and Evans Blue Dye (EBD; right) exclusion in sham-operated B6 mice or 24 hours after Min CI or Ext CI Balb/c → B6 transplantation (N ≥ 5). (B) Histology from Min CI (n = 4) or Ext CI (n = 5) Balb/c → B6 lungs on POD 1. (C) Serum concentrations of indicated cytokines from Min CI or Ext CI Balb/c → B6 transplantations 6 and 24 hours after engraftment (n ≥ 4). (D) Neutrophil numbers in the blood, graft tissue, and BAL from Min CI or Ext CI Balb/c → B6 transplantations on POD 1 (n = 4). (E) BM cells were isolated from Min CI or Ext CI Balb/c → B6 lung transplantations 36 hours after engraftment and 2.5 × 104 cells per plate were assayed in methylcellulose for CFU (left), total number of cells per plate to CFU (center), and CFU (right) differentiated as arising from granulocyte (G), granulocyte-macrophage (GM), and granulocyte-erythroid-macrophage-megakaryocyte (GEMM) progenitors. Data shown are from 1 representative experiment from 4 independently conducted experiments. Data for panels A through E represent the mean ± SEM. *P < .05; **P < .01.
Emergency granulopoiesis. (A) PaO2 (left) and Evans Blue Dye (EBD; right) exclusion in sham-operated B6 mice or 24 hours after Min CI or Ext CI Balb/c → B6 transplantation (N ≥ 5). (B) Histology from Min CI (n = 4) or Ext CI (n = 5) Balb/c → B6 lungs on POD 1. (C) Serum concentrations of indicated cytokines from Min CI or Ext CI Balb/c → B6 transplantations 6 and 24 hours after engraftment (n ≥ 4). (D) Neutrophil numbers in the blood, graft tissue, and BAL from Min CI or Ext CI Balb/c → B6 transplantations on POD 1 (n = 4). (E) BM cells were isolated from Min CI or Ext CI Balb/c → B6 lung transplantations 36 hours after engraftment and 2.5 × 104 cells per plate were assayed in methylcellulose for CFU (left), total number of cells per plate to CFU (center), and CFU (right) differentiated as arising from granulocyte (G), granulocyte-macrophage (GM), and granulocyte-erythroid-macrophage-megakaryocyte (GEMM) progenitors. Data shown are from 1 representative experiment from 4 independently conducted experiments. Data for panels A through E represent the mean ± SEM. *P < .05; **P < .01.
Interaction between neutrophils and DCs promotes alloimmunity
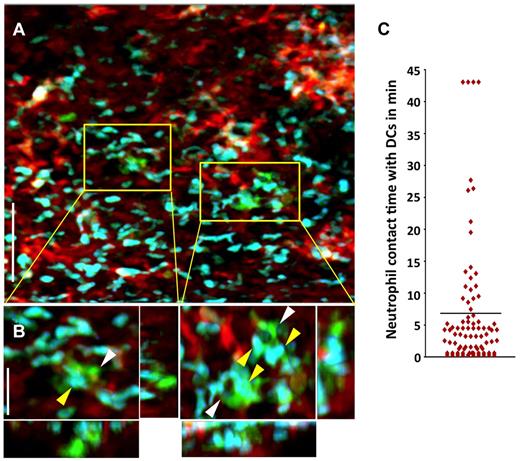
We have recently shown that, unlike the case for other solid organs, alloimmune responses are initiated within lungs independently of secondary lymphoid tissues.9 In particular, T cells cluster around graft-resident donor CD11c+ DCs as early as 30 hours after engraftment.9 Recent work from our laboratory reporting the first description of intravital 2P microscopy of mouse lungs has shown a rapid influx of neutrophils into pulmonary grafts immediately after reperfusion.8 Because neutrophils have also been implicated in directly stimulating DC activation after microbial infection, we investigated whether neutrophils interact with graft-resident DCs using intravital 2P microscopy.16 We observed associations between neutrophils and donor CD11c+ DCs within lung allografts within 1 hour after transplantation, which precedes the time point when we observed interactions between donor-derived DCs and graft-infiltrating T lymphocytes.9 We measured the contact time between donor CD11c+ DCs and graft-infiltrating neutrophils and the mean was nearly 7 minutes, with some associations lasting for the entire observation period. Moreover, some DCs interacted with up to 30 neutrophils during this time span (Figure 2A-C and supplemental Videos 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Neutrophils make prolonged associations with donor-derived graft-resident. (A) Intravital 2P imaging of the distribution of donor DCs (green) and recipient neutrophils (blue) within lung grafts 1 hour after B10.BR CD11c-EYFP → B6 LysM-GFP transplantation. Blood vessels appear red (nontargeted 655-nm quantum dots). Scale bar indicates 60 μm. (B) Zoomed views show several neutrophils (yellow) making contact with CD11c+ DCs (white). Scale bar indicates 15 μm. (C) Measurement of neutrophil contact time with DCs over a 43.5-minute period approximately 1 hour after engraftment. The bar represents the mean neutrophil-DC contact time of 6.8 minutes. Some cells remained associated for the entire imaging period.
Neutrophils make prolonged associations with donor-derived graft-resident. (A) Intravital 2P imaging of the distribution of donor DCs (green) and recipient neutrophils (blue) within lung grafts 1 hour after B10.BR CD11c-EYFP → B6 LysM-GFP transplantation. Blood vessels appear red (nontargeted 655-nm quantum dots). Scale bar indicates 60 μm. (B) Zoomed views show several neutrophils (yellow) making contact with CD11c+ DCs (white). Scale bar indicates 15 μm. (C) Measurement of neutrophil contact time with DCs over a 43.5-minute period approximately 1 hour after engraftment. The bar represents the mean neutrophil-DC contact time of 6.8 minutes. Some cells remained associated for the entire imaging period.
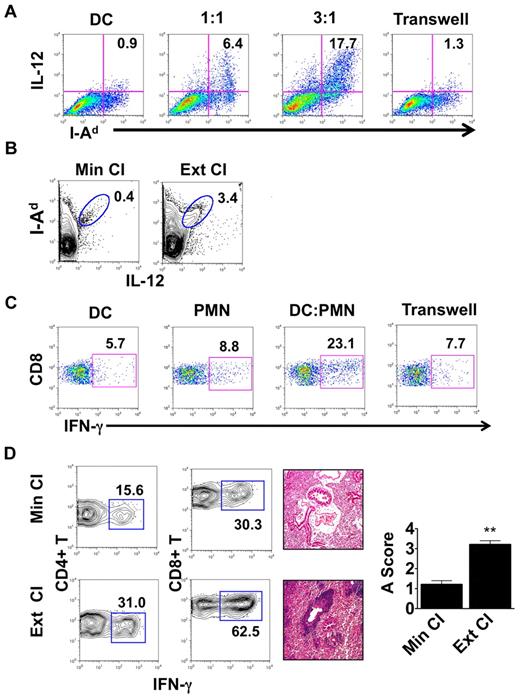
We then set out to assess whether interactions between neutrophils and DCs have functional consequences. We cultured Balb/c BMDCs with neutrophils isolated from the airways of Ext CI B6 → B6 lung transplantations 24 hours after engraftment (Figure 3A). After coculture with neutrophils, IL-12 expression in BMDCs was markedly up-regulated compared with BMDCs cultured alone. We also observed increased MHC class II expression on BMDCs after culture with neutrophils. IL-12 expression could be further enhanced when we increased the ratio of neutrophils to BMDCs, suggesting a quantitative relationship between the level of graft infiltration by neutrophils and the potential of graft-resident DCs to drive alloimmune responses. When neutrophils and BMDCs were separated by a Transwell, there was little change in BMDC IL-12 expression, demonstrating that activation of DCs by neutrophils was dependent on cellular contact. IL-12 production by donor MHC II (I-Ad)–expressing cells within the graft was also enhanced 1 day after transplantation of Ext CI Balb/c lungs into B6 hosts (Figure 3B). Nearly all intragraft IL-12+ I-Ad+ cells expressed CD11c, which is consistent with a DC phenotype (supplemental Figure 1). Next, we examined whether neutrophil-stimulated BMDCs could promote Th1 responses in vivo. We injected neutrophil-stimulated Balb/c BMDCs into B6 mice, and their splenic CD8+ T cells were assessed for recall responses to Balb/c alloantigens 1 week later (Figure 3C). Compared with BMDCs alone, neutrophil-stimulated BMDCs elicited markedly enhanced IFN-γ responses by CD8+ T cells. Consistent with our results for IL-12 production by BMDCs, these augmented recall responses were dependent on direct contact between BMDCs and neutrophils during their initial culture.
Neutrophils from lung recipients activate DCs in a contact-dependent manner. (A) IL-12 and MHC II (I-Ad) expression in Balb/c BMDCs cultured for 24 hours alone or with BAL neutrophils from Ext CI B6 → B6 lung transplantations at a BAL neutrophil to BMDC ratio of 1:1 or 3:1, or separated by a Transwell at a ratio of 3:1. (B) Percent abundance of intragraft IL-12+ I-Ad+ cells in Min CI or Ext CI Balb/c → B6 transplantations on POD 1. (C) IFN-γ expression after stimulation with Balb/c splenocytes in CD8+ T cells isolated from B6 mice 1 week after injection of immature Balb/c BMDCs (DC), BAL neutrophils (PMN) from Ext CI B6 → B6 transplantations, Balb/c BMDCs cultured with PMN, or Balb/c BMDCs cultured with PMN separated by a Transwell. (D) Percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n ≥ 6; left), histology (n ≥ 6; center), and rejection scores (n = 5; right) in costimulation blockade-treated Min CI or Ext CI Balb/c → B6 transplantations on POD 7. Results are shown as means ± SEM. *P < .05; **P < .01. Data for panels A-E are representative of ≥ 3 independent experiments unless otherwise noted.
Neutrophils from lung recipients activate DCs in a contact-dependent manner. (A) IL-12 and MHC II (I-Ad) expression in Balb/c BMDCs cultured for 24 hours alone or with BAL neutrophils from Ext CI B6 → B6 lung transplantations at a BAL neutrophil to BMDC ratio of 1:1 or 3:1, or separated by a Transwell at a ratio of 3:1. (B) Percent abundance of intragraft IL-12+ I-Ad+ cells in Min CI or Ext CI Balb/c → B6 transplantations on POD 1. (C) IFN-γ expression after stimulation with Balb/c splenocytes in CD8+ T cells isolated from B6 mice 1 week after injection of immature Balb/c BMDCs (DC), BAL neutrophils (PMN) from Ext CI B6 → B6 transplantations, Balb/c BMDCs cultured with PMN, or Balb/c BMDCs cultured with PMN separated by a Transwell. (D) Percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n ≥ 6; left), histology (n ≥ 6; center), and rejection scores (n = 5; right) in costimulation blockade-treated Min CI or Ext CI Balb/c → B6 transplantations on POD 7. Results are shown as means ± SEM. *P < .05; **P < .01. Data for panels A-E are representative of ≥ 3 independent experiments unless otherwise noted.
The enhanced inflammatory response associated with extended graft storage raised the possibility that emergency granulopoiesis could adversely affect the ability to induce lung graft acceptance. To address this question, we immunosuppressed B6 recipients of Min CI or Ext CI Balb/c lungs with a costimulatory blockade regimen that we have reported previously to prevent acute lung rejection12 (Figure 3D and supplemental Figure 2A). Seven days after engraftment, Min CI grafts had minimal inflammation, whereas in stark contrast, Ext CI lungs had extensive perivascular and peribronchial infiltrates, the hallmarks of acute lung rejection. We also observed an approximately 2-fold expansion of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in Ext CI compared with Min CI grafts.
G-CSF–mediated granulopoiesis prevents lung allograft acceptance
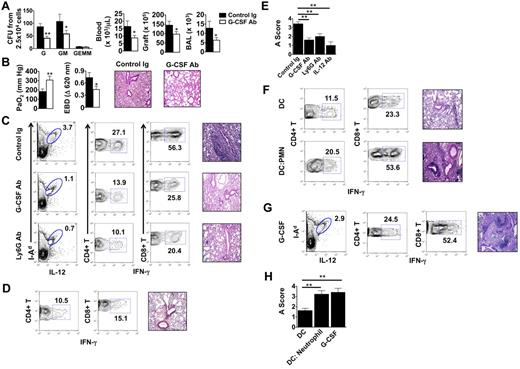
We next assessed the role of G-CSF–mediated emergency granulopoiesis on the fate of lung allografts that had undergone extended graft ischemia. Treatment of B6 recipients of Ext CI Balb/c lungs with G-CSF–neutralizing Ab resulted in significant reductions of neutrophil progenitors in the BM and diminished accumulation of neutrophils in the blood, graft tissue, and airways (Figure 4A). Furthermore, G-CSF blockade led to improved allograft function and attenuated pulmonary edema (Figure 4B), along with a marked reduction of graft-resident IL-12–producing CD11c+I-Ad+ cells and graft-infiltrating IFN-γ+CD4+ and IFN-γ+CD8+ T cells (Figure 4C left and middle panels). Inhibition of emergency granulopoiesis by blocking G-CSF or administration of neutrophil-depleting Abs significantly attenuated rejection in Ext CI grafts (Figure 4C right panels, Figure 4E, and supplemental Figure 2B,D). We next investigated whether IL-12 promotes lung rejection in recipients of Ext CI lungs (Figure 4D-E and supplemental Figure 2C-D). Similar to G-CSF blockade, administration of IL-12-neutralizing Abs to B6 recipients of Ext CI Balb/c lungs reduced the accumulation of graft-infiltrating IFN-γ+CD4+ and IFN-γ+CD8+ T cells in the graft and prevented rejection.
G-CSF blockade promotes the acceptance of lung allografts exposed to extended cold ischemia. (A) BM cells isolated from Ext CI Balb/c → B6 lung transplantations treated with control Ig or anti–G-CSF Ab 36 hours after engraftment and 2.5 × 104 cells per plate were methylcellulose assayed for CFU-G, CFU-GM and CFU-GEMM numbers (n = 4; left). Neutrophil numbers in peripheral blood, lung tissue, and BAL from control Ig or anti–G-CSF Ab–treated Ext CI Balb/c → B6 transplantations 24 hours after engraftment (n = 4; right). (B) Analysis of PaO2 (n = 5; left), exclusion of EBD (center); and histology (n = 5; right) after engraftment. (C) Percent abundance of intragraft IL-12+ I-Ad+ cells on POD 1 (n = 3; left), percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells on POD 7 (n ≥ 4; center) and representative histology (n ≥ 4; right) on POD 7 from control Ig–treated, G-CSF Ab–treated, or Ly6G Ab–treated Ext CI Balb/c → B6 transplantations that received costimulatory blockade. (D) Ext CI Balb/c → B6 transplantations that received costimulatory blockade and IL-12–neutralizing Abs (n = 4) and assessed on POD 7 for the percentage abundance of intragraft IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells (n = 4; left) and representative histology (n = 4; right) (E) Rejection scoring of lung grafts on POD 7 from costimulatory blockade-treated Ext CI Balb/c → B6 recipients that received control Ig, G-CSF, Ly6G, or IL-12 Abs. (F) Percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n ≥ 3; left) and representative histology (n ≥ 3; right) on POD 7 from costimulatory blockade-treated Min CI Balb/c → B6 transplantations that received an IV injection of either 5 × 105 Balb/c BMDCs (DC) or 5 × 105 Balb/c BMDCs activated by coculture with BAL neutrophils from Ext CI B6 → B6 transplantations (DC: Neutrophil). (G) Percent abundance of intragraft IL-12+I-Ad+ cells on POD 1 (n = 3; left), percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n = 4; center) on POD 7 and histology on POD 7 (n = 4; right) from costimulatory blockade–treated Min CI Balb/c → B6 transplantations that received mouse recombinant G-CSF (10 μg) immediately after reperfusion. (H) Rejection scoring on POD 7 for costimulatory blockade–treated Min CI Balb/c → B6 transplantations that received DCs, DC:Neutrophil, or G-CSF. Data for panels A, B, E, and H represent the means ± SEM. *P < .05; **P < .01.
G-CSF blockade promotes the acceptance of lung allografts exposed to extended cold ischemia. (A) BM cells isolated from Ext CI Balb/c → B6 lung transplantations treated with control Ig or anti–G-CSF Ab 36 hours after engraftment and 2.5 × 104 cells per plate were methylcellulose assayed for CFU-G, CFU-GM and CFU-GEMM numbers (n = 4; left). Neutrophil numbers in peripheral blood, lung tissue, and BAL from control Ig or anti–G-CSF Ab–treated Ext CI Balb/c → B6 transplantations 24 hours after engraftment (n = 4; right). (B) Analysis of PaO2 (n = 5; left), exclusion of EBD (center); and histology (n = 5; right) after engraftment. (C) Percent abundance of intragraft IL-12+ I-Ad+ cells on POD 1 (n = 3; left), percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells on POD 7 (n ≥ 4; center) and representative histology (n ≥ 4; right) on POD 7 from control Ig–treated, G-CSF Ab–treated, or Ly6G Ab–treated Ext CI Balb/c → B6 transplantations that received costimulatory blockade. (D) Ext CI Balb/c → B6 transplantations that received costimulatory blockade and IL-12–neutralizing Abs (n = 4) and assessed on POD 7 for the percentage abundance of intragraft IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells (n = 4; left) and representative histology (n = 4; right) (E) Rejection scoring of lung grafts on POD 7 from costimulatory blockade-treated Ext CI Balb/c → B6 recipients that received control Ig, G-CSF, Ly6G, or IL-12 Abs. (F) Percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n ≥ 3; left) and representative histology (n ≥ 3; right) on POD 7 from costimulatory blockade-treated Min CI Balb/c → B6 transplantations that received an IV injection of either 5 × 105 Balb/c BMDCs (DC) or 5 × 105 Balb/c BMDCs activated by coculture with BAL neutrophils from Ext CI B6 → B6 transplantations (DC: Neutrophil). (G) Percent abundance of intragraft IL-12+I-Ad+ cells on POD 1 (n = 3; left), percent abundance of intragraft IFN-γ+CD4+ and IFN-γ+CD8+ T cells (n = 4; center) on POD 7 and histology on POD 7 (n = 4; right) from costimulatory blockade–treated Min CI Balb/c → B6 transplantations that received mouse recombinant G-CSF (10 μg) immediately after reperfusion. (H) Rejection scoring on POD 7 for costimulatory blockade–treated Min CI Balb/c → B6 transplantations that received DCs, DC:Neutrophil, or G-CSF. Data for panels A, B, E, and H represent the means ± SEM. *P < .05; **P < .01.
Finally, we assessed whether neutrophil-stimulated BMDCs could prevent the acceptance of Min CI lung allografts. B6 hosts were primed with Balb/c BMDCs alone or neutrophil-stimulated Balb/c BMDCs, allowed to rest for 7 days, and then engrafted with Balb/c lungs and treated with costimulatory blockade (Figure 4F and supplemental Figure 2E). Unlike recipients that received BMDCs alone, mice that received neutrophil-stimulated BMDCs rejected Balb/c lungs. Graft rejection was associated with an augmented accumulation of IFN-γ+CD4+ and IFN-γ+CD8+ T cells. Similarly, administration of G-CSF abrogated allograft acceptance of Min CI Balb/c lungs after transplantation into B6 hosts and resulted in enhanced graft infiltration with IFN-γ–producing CD4+ and CD8+ T cells (Figure 4G-H).
Neutrophil TNF-α stimulates IL-12 production in DCs
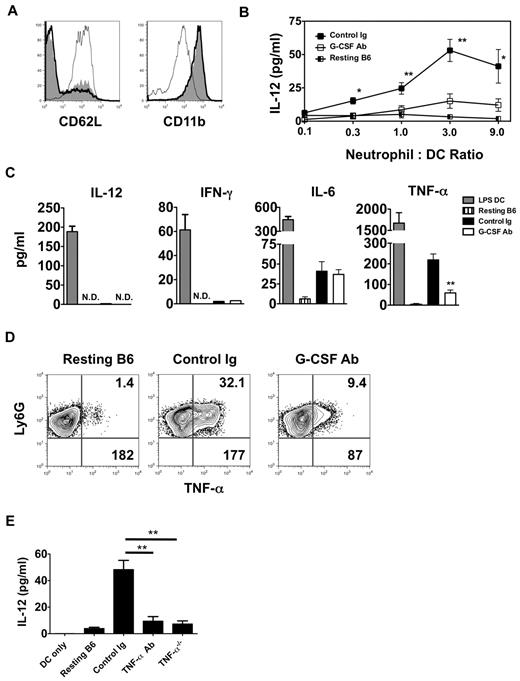
In addition to enhancing granulopoiesis, G-CSF is a critical enhancer of neutrophil activation.17 Moreover, several reports have linked neutrophil activation to DC maturation.16,18 We therefore assessed the activation state of neutrophils isolated from the airways of G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients 24 hours after engraftment (Figure 5A). Examination of CD11b and CD62L expression on airway-infiltrating neutrophils from these mice revealed essentially no difference in activation compared with control Ig–treated lung recipients. However, neutrophils from G-CSF Ab–treated lung recipient airways were poor at stimulating IL-12 production in BMDCs compared with neutrophils isolated from either control Ig–treated lung recipient airways or the BM from resting B6 mice (Figure 5B). We next measured the accumulation of Th1-associated cytokines in cultures with neutrophils isolated from the airways of G-CSF Ab- and control Ig–treated Ext CI Balb/c → B6 lung recipients (Figure 5C). Neutrophils from both groups produced little IL-12 or IFN-γ, but equivalent amounts of IL-6. However, TNF-α accumulation was greatly reduced in cultures derived from G-CSF Ab–treated lung recipients. Moreover, G-CSF blockade reduced the percentage abundance of TNF-α+ neutrophils and cellular TNF-α expression levels in airway neutrophils from Ext CI Balb/c lung grafts (Figure 5D and supplemental Figure 2F).
TNF-α from lung recipient neutrophils drives IL-12 expression in DCs. (A) Representative histogram (n = 4) of CD62L and CD11b expression of either neutrophils isolated from the airway of control Ig–treated (thick line) and G-CSF Ab-treated (solid line) Ext CI Balb/c → B6 lung recipients on POD 1 or neutrophils from resting B6 mouse BM (thin line). (B) Airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients on POD1 or neutrophils from resting B6 mouse BM were cocultured with BMDCs in the indicated neutrophil to DC ratios for 24 hours and then supernatants were assessed for the accumulation of IL-12. Results are representative of 2 independently performed experiments. (C) BMDCs were stimulated with 10 ng/mL of LPS for 24 hours (LPS DC) or POD 1 airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients or neutrophils from resting B6 mouse BM were cultured for 24 hours and then supernatants were analyzed for IL-12, IFN-γ, IL-6, and TNF-α. Results are representative of 3 independently performed experiments. (D) Intracellular TNF-α expression from resting B6 mouse BM neutrophils or airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients on POD 1. Representative results from 4 independent experiments are shown, in which the upper right number indicates the percentage abundance of TNF-α+ cells and the lower right number is the mean fluorescence intensity of TNF-α expression. (E) IL-12 accumulation in the supernatants of BMDC culture or BMDC coculture with resting B6 neutrophils, POD 1 airway neutrophils from Ext CI Balb/c → B6 lung recipients, treated with control Ig or TNF-α Abs, or POD 1 airway neutrophils from Ext CI Balb/c → B6 TNF-α−/− lung recipients. Data are representative of 2 independent experiments. Data for panels B and E represent the means ± SEM. *P < .05; **P < .01.
TNF-α from lung recipient neutrophils drives IL-12 expression in DCs. (A) Representative histogram (n = 4) of CD62L and CD11b expression of either neutrophils isolated from the airway of control Ig–treated (thick line) and G-CSF Ab-treated (solid line) Ext CI Balb/c → B6 lung recipients on POD 1 or neutrophils from resting B6 mouse BM (thin line). (B) Airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients on POD1 or neutrophils from resting B6 mouse BM were cocultured with BMDCs in the indicated neutrophil to DC ratios for 24 hours and then supernatants were assessed for the accumulation of IL-12. Results are representative of 2 independently performed experiments. (C) BMDCs were stimulated with 10 ng/mL of LPS for 24 hours (LPS DC) or POD 1 airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients or neutrophils from resting B6 mouse BM were cultured for 24 hours and then supernatants were analyzed for IL-12, IFN-γ, IL-6, and TNF-α. Results are representative of 3 independently performed experiments. (D) Intracellular TNF-α expression from resting B6 mouse BM neutrophils or airway neutrophils from control Ig–treated or G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients on POD 1. Representative results from 4 independent experiments are shown, in which the upper right number indicates the percentage abundance of TNF-α+ cells and the lower right number is the mean fluorescence intensity of TNF-α expression. (E) IL-12 accumulation in the supernatants of BMDC culture or BMDC coculture with resting B6 neutrophils, POD 1 airway neutrophils from Ext CI Balb/c → B6 lung recipients, treated with control Ig or TNF-α Abs, or POD 1 airway neutrophils from Ext CI Balb/c → B6 TNF-α−/− lung recipients. Data are representative of 2 independent experiments. Data for panels B and E represent the means ± SEM. *P < .05; **P < .01.
Because TNF-α can potentiate IL-12 gene transcription in DCs, we next investigated whether TNF-α derived from neutrophils stimulated IL-12 expression in DCs.19,20 Airway neutrophils isolated from Ext CI Balb/c grafts of either B6 wild-type or TNF-α–deficient recipients were assessed for their ability to stimulate IL-12 production after coculture with BMDCs (Figure 5E). Neutrophils from TNF-α–deficient lung recipients induced significantly less IL-12 expression in BMDCs compared with their wild-type B6 counterparts. Furthermore, the addition of TNF-α–neutralizing Abs to B6 airway neutrophil BMDC cocultures blunted IL-12 production to levels comparable to cocultures of BMDCs with TNF-α–deficient neutrophils. Therefore, our results indicate that TNF-α expressed by graft-infiltrating neutrophils drives IL-12 production in DCs.
Neutrophil degranulation enhances TNF-α–mediated IL-12 production by DCs
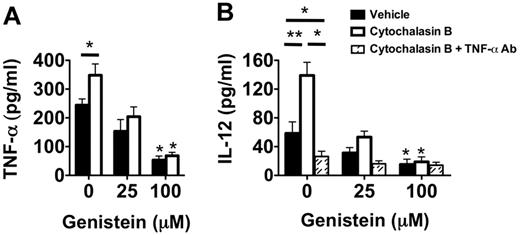
These high levels of intracellular TNF-α in Ext CI Balb/c → B6 recipient airway neutrophils raised the possibility that degranulation plays an important role in stimulating DC IL-12 production. Inhibition of degranulation with the exocytosis inhibitor genistein21 greatly reduced the release of TNF-α by cultured airway neutrophils from Ext CI Balb/c → B6 lung recipients (Figure 6A). Conversely, the degranulation-enhancing agent cytochalasin B21 significantly augmented TNF-α release by airway neutrophils. To determine the role of degranulation in DC IL-12 production, we added cytochalasin B–treated airway neutrophils to BMDC cultures (Figure 6B). IL-12 accumulation was significantly enhanced in cytochalasin B–treated neutrophil BMDC cocultures. Cytochalasin B–mediated IL-12 production could be completely inhibited by the addition of TNF-α–neutralizing Abs, indicating that this effect is primarily controlled through TNF-α. Moreover, treatment of airway neutrophils with genistein inhibited IL-12 production in both vehicle-treated and cytochalasin B–treated cocultures. In addition, reduction of the generation of reactive oxygen species by airway neutrophils with the NAPDH oxidase inhibitor VAS287022 had little effect on IL-12 production in BMDC coculture (supplemental Figure 3). Therefore, our data indicate that graft-infiltrating neutrophil degranulation plays a prominent role in controlling IL-12 production in DCs.
Neutrophil degranulation increases TNF-α–mediated IL-12 expression in DCs. (A) Supernatant TNF-α accumulation of vehicle-treated, cytochalasin B-treated, genistein-treated, or genistein + cytochalasin B–treated POD1 airway neutrophils from Ext CI Balb/c → B6 lung recipients. Results are representative of 3 independent experiments. (B) Supernatant IL-12 accumulation in BMDC cocultures with vehicle-treated, cytochalasin B-treated, genistein-treated, or genistein + cytochalasin B–treated POD1 airway neutrophils from Ext CI Balb/c → B6 lung recipients cultured in the absence or presence of TNF-α Abs. Results are representative of 3 independent experiments. Data for panels A and B represent the mean ± SEM. *P < .05; **P < .01.
Neutrophil degranulation increases TNF-α–mediated IL-12 expression in DCs. (A) Supernatant TNF-α accumulation of vehicle-treated, cytochalasin B-treated, genistein-treated, or genistein + cytochalasin B–treated POD1 airway neutrophils from Ext CI Balb/c → B6 lung recipients. Results are representative of 3 independent experiments. (B) Supernatant IL-12 accumulation in BMDC cocultures with vehicle-treated, cytochalasin B-treated, genistein-treated, or genistein + cytochalasin B–treated POD1 airway neutrophils from Ext CI Balb/c → B6 lung recipients cultured in the absence or presence of TNF-α Abs. Results are representative of 3 independent experiments. Data for panels A and B represent the mean ± SEM. *P < .05; **P < .01.
Neutrophil plasma membrane–associated TNF-α drives IL-12 production in DCs
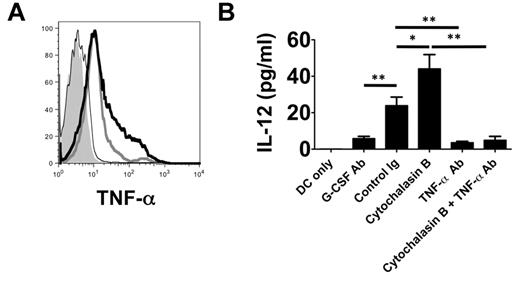
TNF-α associates with the plasma membrane of neutrophils after LPS stimulation.16 Because we observed that neutrophil contact with BMDCs is required to induce their production of IL-12 (Figure 3A), we hypothesized that TNF-α may be expressed on the surface of neutrophils from lung recipients (Figure 7A). In neutrophils isolated from the airways of Ext CI Balb/c → B6 recipients, we observed marked cell-surface expression of TNF-α, which could be further enhanced by treatment with cytochalasin B. In contrast, TNF-α expression was virtually absent on the surface of airway neutrophils isolated from G-CSF Ab–treated Ext CI Balb/c → B6 recipients. We next investigated whether cell surface–associated TNF-α could induce IL-12 expression in DCs (Figure 7B). We assessed IL-12 production after culturing BMDCs with plasma membranes isolated from neutrophils derived from the airways of control Ig- and G-CSF Ab–treated lung recipients, respectively. Plasma membranes from control Ig–treated neutrophils were significantly better at stimulating DC IL-12 production than G-CSF–Ab–treated neutrophil plasma membranes. Stimulation of airway neutrophils from control Ig–treated lung recipients with cytochalasin B further increased the production of IL-12. Finally, TNF-α blockade reduced BMDC IL-12 production induced by plasma membranes from stimulated or unstimulated neutrophils of control Ig–treated lung recipients to levels that were similar to those seen after stimulation with neutrophil plasma membranes from G-CSF Ab–treated hosts.
TNF-α is associated with the neutrophil plasma membranes and stimulates IL-12 expression in DCs. (A) Histogram of surface TNF-α expression on POD 1 airway neutrophils from unstimulated (thick gray line) or cytochalasin B–stimulated (thick black line) control Ig–treated Ext CI Balb/c → B6 lung recipients or (thin black line) G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients or (shaded) Ext CI Balb/c → B6 TNF-α−/− lung recipients. Results are representative of 2 independent experiments. (B) BMDC culture (DCs only) or BMDCs cocultured with unstimulated (control Ig) or cytochalasin B–stimulated (cytochalasin B) POD 1 Ext CI Balb/c → B6 lung recipient airway neutrophil plasma membranes in the absence or presence of TNF-α Ab (TNF-α Ab or cytochalasin B + TNF-α Ab, respectively) or with POD 1 G-CSF Ab–treated Ext CI Balb/c → B6 lung recipient airway neutrophil plasma membranes (G-CSF Ab). One day later, IL-12 accumulation was assessed in culture supernatants. Results are representative of 2 independent experiments. Data represent the means ± SEM. *P < .05; **P < .01.
TNF-α is associated with the neutrophil plasma membranes and stimulates IL-12 expression in DCs. (A) Histogram of surface TNF-α expression on POD 1 airway neutrophils from unstimulated (thick gray line) or cytochalasin B–stimulated (thick black line) control Ig–treated Ext CI Balb/c → B6 lung recipients or (thin black line) G-CSF Ab–treated Ext CI Balb/c → B6 lung recipients or (shaded) Ext CI Balb/c → B6 TNF-α−/− lung recipients. Results are representative of 2 independent experiments. (B) BMDC culture (DCs only) or BMDCs cocultured with unstimulated (control Ig) or cytochalasin B–stimulated (cytochalasin B) POD 1 Ext CI Balb/c → B6 lung recipient airway neutrophil plasma membranes in the absence or presence of TNF-α Ab (TNF-α Ab or cytochalasin B + TNF-α Ab, respectively) or with POD 1 G-CSF Ab–treated Ext CI Balb/c → B6 lung recipient airway neutrophil plasma membranes (G-CSF Ab). One day later, IL-12 accumulation was assessed in culture supernatants. Results are representative of 2 independent experiments. Data represent the means ± SEM. *P < .05; **P < .01.
Discussion
Mechanisms that promote alloimmunity in lung recipients who suffer from ischemia-reperfusion injury–mediated primary graft dysfunction remain undefined. It has been repeatedly demonstrated that lung recipients with severe grades of primary graft dysfunction have elevated levels of IL-12 in their BAL, suggesting the potential for Th1 responses.23,24 We and others have shown that primary graft dysfunction is associated with neutrophil influx into lung grafts.4,8,25 In addition to well-defined neutrophil chemoattractants such as IL-8,25 several reports indicate that G-CSF directly promotes neutrophil recruitment to inflammatory sites, including the lung.26,27 In this context, G-CSF is thought to play several complementary roles, which include augmenting CXCR2-mediated neutrophil chemotaxis,28 enhancing the mobilization of neutrophils from the BM into the blood,29 and stimulating BM granulopoiesis.30 In the present study, we observed that augmented G-CSF levels are associated with high intragraft IL-12+ DC and Th1-cell abundance in rejecting Ext CI lung recipients. G-CSF blockade prevented the accumulation of these cells. We also showed that IL-12 is a critical regulator of the survival of Ext CI lung grafts, because blockade with IL-12–specific Abs blunted Th1-cell intragraft accumulation and prevented acute rejection. Therefore, our data show that G-CSF adversely affects the survival of lung allografts through augmentation of Th1 alloimmunity, a result that was corroborated by our finding that treatment of Min CI lung recipients with G-CSF prevented graft acceptance.
Interestingly, G-CSF may also target mechanisms that promote Th1 responses that are independent of neutrophil production. In a mouse model of allogeneic BM transplantation, G-CSF administration can activate donor DCs both to stimulate natural killer T cells to produce IFN-γ and to prime effector CD8+ T cells to promote GVHD.31 A similar report demonstrated that G-CSF could also directly stimulate natural killer T cells to promote Th1 responses.32 Therefore, it remains possible that G-CSF may partly promote Th1 responses in a manner independent of neutrophil-mediated IL-12 production by DCs after lung transplantation. In addition, G-CSF administration may have direct effects on CD4+ T cells, mainly biasing them toward Th2 polarization33-35 in cases where this agent is used to harvest hematopoietic progenitors. The discordance between these previous observations and our results may be explained by temporal differences in G-CSF concentration. After lung transplantation G-CSF serum, levels rise sharply after engraftment, but decline rapidly within 18 hours after reperfusion.4 Accordingly, in our present studies, G-CSF was administered as a single bolus at the time of transplantation to better model the kinetics of G-CSF expression in Ext CI lung recipients. In contrast, when G-CSF is used to mobilize hematopoietic progenitors, it is generally given over the course of up to 5 days or it is PEGylated to increase stability, potentiating prolonged exposure to T cells within the blood and BM.35,36 Therefore, it remains likely that during lung transplantation, direct G-CSF exposure to T cells is relatively transient compared with its use as an agent to promote hematopoietic progenitor isolation.
There is controversy as to how encounters between neutrophils and DCs within secondary lymphoid organs regulate adaptive immunity. Some studies have suggested that neutrophils can stimulate splenic DC maturation after microbial infection and that these direct interactions promote Th1 responses.7,16 In contrast, a more recent study using 2P microscopy to examine the effects of adjuvant-enhanced antigen presentation demonstrated that neutrophil encounters with DCs inhibited subsequent T-cell priming.37 Therefore, it may be a combination of the nature of the stimulus and the impact of the local environment that ultimately determines the outcome of neutrophil-DC interactions.
We and others have shown that the lung provides a suitable environment for the initiation of adaptive immune responses.9,38,39 These observations have led us to better define cellular interactions within this organ that shape the outcome of immune-mediated processes. Because earlier studies have reported that neutrophil graft infiltration is associated with lung rejection, we hypothesized that lung alloimmunity could be regulated by interactions between neutrophils and DCs within the graft.40,41 Using intravital 2P microscopy, we observed frequent and often stable interactions between graft-infiltrating neutrophils and lung-resident, donor-derived DCs. Because our previous work demonstrated that T cells cluster around donor DCs 1 day after transplantation, and that acute rejection is associated with Th1 differentiation,42,43 these findings suggest that graft-infiltrating neutrophils enhance Th1 alloimmunity through stimulation of donor DCs. Additional support for this notion is provided by our observation that depletion of neutrophils leads to a reduction of IL-12+ DCs and IFN-γ+ T cells in Ext CI allografts. Moreover, airway neutrophils can stimulate BMDCs to express IL-12 in a contact-dependent manner and enhance their capacity to promote Th1 responses.
Earlier reports had suggested that neutrophil activation is critical to stimulating DC-maturation responses.7,18 Surprisingly, the expression patterns of CD62L and CD11b indicated that activated-state neutrophils from G-CSF Ab–treated lung recipients were as inefficient at stimulating at IL-12 gene expression in BMDCs as neutrophils from resting mice. This observation led us to investigate whether there were deficits in Th1-promoting cytokines in G-CSF Ab–treated lung recipient neutrophils. In an analysis of a panel of Th1 cytokines, TNF-α expression was significantly lower in airway neutrophils in G-CSF Ab–treated lung recipients. This observation extends previous reports showing that G-CSF stimulates the release of TNF homologs such as the B-lymphocyte stimulator from neutrophils.44,45 We demonstrate that IL-12 production in DCs is predominantly controlled by TNF-α expression in lung recipient neutrophils. This finding is supported by 2 previous reports linking LPS-mediated TNF-α expression in neutrophils to DC-maturation responses.16,18 TNF-α has both soluble and membrane-associated forms,46 which raised the possibility that neutrophils can drive TNF-α–mediated DC IL-12 production in lung grafts by either contact-dependent or contact-independent routes. We observed both types of TNF-α expression in control-Ig–treated lung recipient neutrophils, whereas in contrast, we did not detect membrane-associated TNF-α after G-CSF Ab treatment. The importance of plasma membrane–associated TNF-α is underscored by our finding that TNF-α–neutralizing Abs prevent plasma membranes from control Ig–treated lung recipient neutrophils from stimulating DC IL-12 production.
To our knowledge, G-CSF–dependent expression of TNF-α on the surface of neutrophils has not been reported previously. It is well established that G-CSF potentiates the activation of exocytosis pathways that promote the mobilization of granulocyte effector molecules such as proteases, gelatinases, and cytokines, including TNF-α.47 Therefore, it is possible that G-CSF promotes the accumulation of membrane-associated TNF-α through stimulating the fusion of TNF-α–containing granules with the plasma membrane. We were able to significantly blunt TNF-α–mediated IL-12 expression in DCs by inhibiting degranulation with genistein. Furthermore, enhancing degranulation with cytochalasin B increased neutrophil plasma membrane–associated TNF-α expression and neutrophil plasma membrane–mediated IL-12 production in DCs. We also observed a small amount of neutrophil-mediatedDC IL-12 expression that was neither dependent on degranulation or TNF-α expression, suggesting the existence of additional mechanisms that neutrophils use to stimulate DC maturation. One possible mechanism is neutrophil elastase release, which has been shown to activate TLR4,48 a potent activator of IL-12 expression in DCs.49
In addition to directly controlling DC activation, neutrophils may also regulate adaptive immune responses by controlling the entry of effector T lymphocytes into inflamed tissue. In a mouse cardiac allograft model, neutrophils were shown to promote the accumulation of alloantigen-primed T cells, leading to accelerated heart rejection possibly by inducing the graft expression of T-cell attractants such as IFN-γ–inducible protein-10 and monokine induced by IFN-γ.50,51 In contrast, results from a later study suggested that neutrophils may inhibit the transmigration of T cells into inflamed sites by elastase-mediated deactivation of endothelium-associated CXCL12.52 We have recently demonstrated that monocytes regulate the transendothelial migration of neutrophils into lung grafts.8 Future studies using intravital 2P microscopy will be helpful in defining how neutrophils regulate the trafficking of other cell populations within lungs.
The current study identifies a link between emergency granulopoiesis and alloimmunity after lung transplantation. Our findings in the mouse mirror events in human lung transplantation, where ischemia reperfusion injury causes early graft dysfunction and affects alloimmunity, resulting in worsened long-term survival.53,54 The results of this study support the concept that inhibition of G-CSF–mediated emergency granulopoiesis may be a promising therapeutic strategy with which to decrease both short-term morbidity and blunt alloimmune responses. In addition, because large numbers of neutrophils infiltrate lungs under a wide variety of inflammatory conditions,55 and adaptive immune responses can be generated within lungs,56 our observations are of potentially high clinical relevance for a broad spectrum of immune-mediated pulmonary diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Christine T. Pham of the Division of Rheumatology and Dr Daniel C. Link of the Division of Hematology and Oncology at Washington University School of Medicine for their advice in the preparation of this manuscript.
D.K. and A.E.G. were supported by a grant sponsored by The National Heart, Lung and Blood Institute (1R01HL094601) and the Barnes Jewish Research Foundation.
National Institutes of Health
Authorship
Contribution: D.K. designed the research and wrote the manuscript; S.S. W.L., M.O., and S.Y. performed the lung transplantations; R.N. and M.J.M. performed the intravital 2P microscopy and analyzed the data; J.Z., M.I., H.J.H., K.A.T., and A.S.K. performed the research; J.H.R. analyzed the histology; and A.E.G. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew E. Gelman, Assistant Professor of Surgery, Pathology & Immunology, Campus Box 8234, 660 South Euclid Ave, Washington University, St Louis, MO 63110-1013; e-mail: gelmana@wudosis.wustl.edu.