Abstract
Millions of lymphocytes enter and exit mammal lymph nodes (LNs) each day, accessing the parenchyma via high endothelial venules (HEVs) and egressing via lymphatics. Despite this high rate of cellular flux and the many entry and exit sites within a given LN, the number of lymphocytes present in a resting LN is extraordinary stable over time, raising the question of how this steady-state is maintained. Here we have examined the anatomic details of lymphocyte movement in HEVs, finding that HEVs create pockets within which lymphocytes reside for several minutes before entering the LN proper. The function of these pockets was revealed in experiments performed under conditions in which lymphocyte egress from the LN was compromised by any of several approaches. Under such conditions, the HEVs pockets behaved as “waiting areas” in which lymphocytes were held until space was made available to them for entry into the parenchyma. Thus, rather than being simple entry ports, HEVs act as gatekeepers able to stack, hold and grant lymphocytes access to LN parenchyma in proportion to the rate of lymphocyte egress from the LN, enabling the LN to maintain a constant steady-state cellularity while supporting the extensive cellular trafficking necessary for repertoire scanning.
Introduction
Despite the constant entry and exit of lymphocytes in the noninflamed state, LNs manage to maintain their cellularity over time, indicating the existence of tightly regulated control mechanisms that balance access and egress of cells.1 Naive lymphocytes enter LNs via HEVs and exit via lymphatic vessels.2 The number of HEVs and lymphatic exit sites in an individual LN is quite high, with these sites showing substantial topographic separation throughout the LN. This raises the question of how a LN manages lymphocyte traffic at these many dispersed sites to maintain homeostasis, a critical issue because LNs provide key survival signals such as IL-7 and BAFF (B-cell activating factor) to T and B cells, respectively.3,4 As a resting LN is believed to secrete defined amounts of such survival signals, increases in lymphocyte number beyond that properly supported by the survival factors available could lead to cell death and repertoire contraction. Likewise, too few cells would limit the number of specific cells available at any time for antigen-driven activation, diminishing the efficiency of immune responses. Interestingly, mice grafted with multiple thymi do not harbor massively enlarged LNs despite high numbers of circulating lymphocytes, suggesting that LNs auto-regulate the number of cells they house and nourish.5,6 Altogether, these observations suggest that LNs adapt lymphocyte entry and exit fluxes to constantly host a fixed number of lymphocytes under steady-state conditions. In this study, we investigate this possibility and demonstrate that, in addition of being passive entry doors for lymphocytes, HEVs are traffic control checkpoints able to create “waiting areas” in which lymphocytes accumulate and are held until space is made available to them in the parenchyma as a consequence of lymphocytes egress. This simple scheme provides an elegant way for the LN to maintain a constant cell number while supporting the high flux of cells needed for effective utilization of the adaptive immune repertoire.
Methods
Mice
C57BL/6, RAG2°/°, actin-CFP,7 Ubiquitin-Cre EsR18 , and C57BL/6 ubiquitin-GFP mice were purchased from The Jackson Laboratory and maintained in the CIML and/or Skirball animal facilities. Ubiquitin-Cre EsR1 were crossed with S1P1flox/− mice kindly provided by R. L. Proia.9 For the generation of chimeras, C57BL/6 RAG2°/° ubiquitin-GFP mice were γ-irradiated (twice with 500 rads) from a cesium source and were reconstituted with various mixtures of bone marrow cells indicated in the main text (minimum of 2 × 106 bone marrow cells per mouse). Chimeras were used at 8 weeks after reconstitution. All procedures performed on animals in this study have been approved by the ethical committee on animal research of Marseille (N°1-03112009, France).
Antibodies
RA3-6B2 antibody specific for B220, 17A2 specific for the CD3 complex, Meca-79 specific for PNAd, 3E2 antibody specific for ICAM-1, MEL-14 specific for CD62L were purchased from BD Biosciences Pharmingen. ERTR-7 and Desmin antibodies were purchased from Acris Antibodies. These antibodies were visualized by direct coupling to Pacific blue, allophycocyanin, Alexa Fluor–488, –568, –647, or through the use of Alexa Fluor–488, –568, –647 or -biotin coupled secondary antibodies. Nuclear staining was performed with Sytox 63 (Invitrogen).
Immunostaining
LNs were harvested and fixed in a 0.05M phosphate buffer containing 0.1M L-lysine (pH 7.4), 2 mg/mL NaIO4, and 10 mg/mL paraformaldehyde (PLP) for 12 hours, then washed in phosphate buffer and dehydrated in 30% sucrose in phosphate buffer. LNs were snap frozen in Tissue-Tek (Sakura Finetek). Twenty-micrometre frozen sections were cut and then stained with the indicated antibodies as previously described.10 Immunofluorescence confocal microscopy was performed with a Leica SP5 confocal microscope. Separate images were collected for each fluorochrome and overlaid to obtain a multicolor image. Final image processing was performed with Imaris Version 7.2.3 software (Bitplane) and Adobe Photoshop.
Homing assay
Total cells were purified from the LNs and spleens of WT mice and stained with either CMFDA (0.5μM) or CMTMR (2μM; Invitrogen) at 37°C for 15 minutes. The indicated numbers of cells were adoptively transferred intravenously. One hour later, blood, auricular and inguinal LNs were harvested.
Induction of lymphocyte sequestration in LNs on FTY720 and SEW-2871 treatment
When indicated, mice received either 100 μg of FTY720 (Cayman Chemical Company) intraperitoneally or 1.2 mg SEW-2871 (Cayman Chemical Company) gavage. Experiments were conducted 36 (FTY-720) or 8 (SEW-2871) hours later, at the peak of lymphopenia.
Tamoxifen treatment of Ubi-cre ESR1 × S1P1flox/− chimera
Animals were treated with 2 mg of 4-hydroxy-tamoxifen intraperitoneally (Sigma-Aldrich) for 3 consecutive days. Mice were used 9 days after the first injection of tamoxifen.
Quantification of lymphocyte densities inside and outside LN HEVs
Immunofluorescence images were segmented into HEVs and the rest of the LN according to PNAd staining. As PNAd is present on the luminal surface of HEV ECs, HEVs areas were defined as 20 μm concentric rings centered on PNAd labeled structures. The numbers of pixels of each area as well as the numbers of adoptively transferred lymphocytes (CMTMR+) present in each area were measured using ImageJ Version 1.44R software (National Institutes of Health). For each LN slice, the densities of CMTMR+ lymphocytes inside HEVs were divided by the densities of CMTMR+ lymphocytes outside HEVs. A small ratio reflects the cells ability to efficiently gain access to the LN parenchyma while an elevated ratio reflects a delay in this process. For each condition, a minimum of 3 different LNs (> 3 different sections per LN) and 1000 cells were counted per mouse. P values were calculated using 2-tailed unpaired t test.
Morphometric analysis of HEV
The height of the endothelium of HEVs was measured in LN sections obtained from RAG-2°/°ubiquitin-GFP mice using a protocol previously described.11 In brief, the outer contour length of GFP+ HEVs and the area covered by GFP+ HEV ECs (including the HEV pockets) were measured with ImageJ software (National Institutes of Health). Endothelium-height was calculated as HEV-surface-area divided by HEV-contour-length, and expressed in arbitrary units.
Electron microscopy
TEM.
PLP-fixed LNs were rinsed in 0.1M phosphate buffer and postfixed for 1 hour in OsO4. LNs were then rinsed in water, dehydrated through graded concentrations of acetone (50, 70, 95, 100% pure, 3 times) before incubation for several hours in a 1:1 vol/vol acetone-Epon mixture. LNs were then incubated overnight in pure Epon before final embedding in Epon. Eighty millimeter thick LN sections were generated using a LEICA Ultracut S ultramicrotome. Sections were stained with uranyl acetate followed by lead citrate and were imaged using a CM12 Philips microscope.
SEM.
PLP-fixed LNs were rinsed in 0.1M phosphate buffer and immersed for 1 hour in a 20% glycerol solution. LNs were then frozen in liquid nitrogen and freeze-cracked before being dehydrated through graded concentrations of ethanol. LNs were desiccated with hexamethyldisilazane and affixed on aluminum stubs with adhesive, coated with gold-palladium, and examined in a SEM (JEOL 6700F, Japan) microscope with an accelerating voltage of 3 kV.
2-P microscopy of lymphocytes immigration in HEVs.
The right popliteal LN of anesthetized ubiquitin-RAG2°/°GFP mice reconstituted with actin-CFP bone marrow cells (2.5% isofluorane in an air/O2 mix) was surgically exposed and imaged with a Zeiss LSM-7 MP system fitted with a 20× water immersion lens (NA = 1.0, Plan-Apochromat, Zeiss). The 2P laser was a Spectra-physics Maï-taï Deepsee tuned to 860 nM. Singles slices of shallow HEVs were imaged between 50-120μM below the capsule and repeated every 4 seconds to create 3D datasets (x, y, and time) that were then processed with Imaris software (Bitplane) and Adobe After Effects (Adobe).
Results
Lymphocytes accumulate in “pockets” below endothelial cells
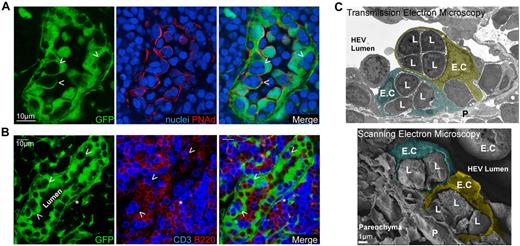
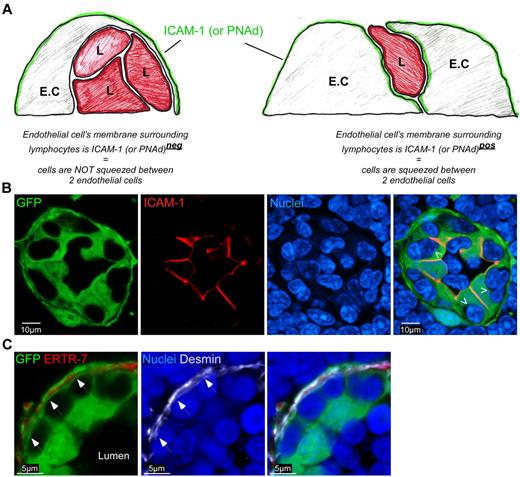
As the entry site for circulating blood lymphocytes, we hypothesized that HEVs may play a key role in regulating resting LNs cellularity by coupling lymphocyte entry with the rate of exit across lymphatic endothelium. To test this hypothesis, we first generated chimeric mice in which HEVs (and other stromal cells) express the green fluorescent protein (GFP). RAG2°/° ubiquitin promoter-GFP transgenic animals were irradiated and reconstituted with wild-type bone marrow cells. Chimeric LNs were harvested, stained for peripheral node addressin (PNAd) and nuclei (Figure 1A) or for CD3 and B220 (Figure 1B), then analyzed by confocal microscopy. As expected and previously observed, all PNAd+ HEVs were GFP+ and harbored typical cobblestone endothelial shapes with numerous embedded lymphocytes.12 Interestingly, a close examination revealed that T and B cells were frequently packed together into HEV pockets and that these structures were able to house up to 4-5 lymphocytes (Figure 1).13,14 Using electron microscopy, we confirmed these results and observed that lymphocytes nested in these pockets were separated from the HEV lumen via a very thin membrane of the HEV endothelial cell (EC; Figure 1C). To determine whether these cells were localized between 2 ECs or within a true pocket formed by single ECs, we took advantage of the asymmetry of HEV ECs. HEVs ECs express molecules such as intercellular adhesion molecule-1 (ICAM-1) on their luminal/lateral face (Figure 2A).15 If the lymphocytes embedded in these pockets were localized between 2 ECs, one would expect them to be entirely surrounded by the ICAM-1 staining of the 2 adjacent ECs membranes (Figure 2A right panel). On the other hand, if the lymphocytes present in these pockets were held by a single EC, the inner membrane of this EC should surround them and hence not be positive for ICAM-1 (Figure 2A left panel). Using such strategy, we determined that lymphocytes nested in these pockets were not localized between 2 HEV ECs (Figure 2B). To distinguish between a situation in which lymphocytes are retained within ECs (emperipolesis) and a situation in which they are nested below ECs, we stained the chimeric LN sections with anti-Desmin and ERTR-7, 2 Abs, respectively specific for myofibroblasts and the conduit system secreted and ensheathed by these cells. Confocal images of HEVs revealed that lymphocytes nested in HEV pockets were localized between the membrane of GFP+ Desmin− EC and the membrane of the GFP+ Desmin+ myofibroblasts that ensheath the ERTR-7 conduit system. Altogether, these results suggest that lymphocytes nested in lymphocytes pockets do not accumulate within ECs but below them (Figure 2C).
HEVs create lymphocyte pockets. (A-B) Confocal images of a LN section from a RAG-2°/°ubiquitin-GFP chimera (green) stained for PNAd (red), sytox 63/nuclei (blue; A), or CD3 (blue) and B220 (red; B) expression, showing the individual HEV ECs and their “pockets” (arrowheads) containing lymphocytes. (*) = Perivascular channel. (C) Representative SEM and TEM pictures of HEV ECs obtained from WT LN sections. Images were pseudocolored to help delineate lymphocytes (L) and endothelial cells (E.C). Data are representative of 3 different experiments (2 mice per experiment, ∼ 20 analyzed HEVs per experiment).
HEVs create lymphocyte pockets. (A-B) Confocal images of a LN section from a RAG-2°/°ubiquitin-GFP chimera (green) stained for PNAd (red), sytox 63/nuclei (blue; A), or CD3 (blue) and B220 (red; B) expression, showing the individual HEV ECs and their “pockets” (arrowheads) containing lymphocytes. (*) = Perivascular channel. (C) Representative SEM and TEM pictures of HEV ECs obtained from WT LN sections. Images were pseudocolored to help delineate lymphocytes (L) and endothelial cells (E.C). Data are representative of 3 different experiments (2 mice per experiment, ∼ 20 analyzed HEVs per experiment).
HEV pockets are plastic structures. (A) The right panel in the diagram represents a situation in which lymphocytes are not engulfed within pockets but simply migrating between 2 ECs while the left panel represents lymphocytes present in a true structured pocket underneath the HEV. L = Lymphocyte. (B) Confocal image of a LN section from a ubiquitin-GFP chimera (green) stained for ICAM-1 (red) and sytox 63/nuclei (blue) Arrowheads indicate the inner membrane of the EC directly in contact with lymphocytes. (C) Confocal image of a LN section from a ubiquitin-GFP chimera (green) stained for ERTR-7 (red), Desmin (white) and sytox 63/nuclei (blue). Arrows indicate the membranes of myofibroblasts/pericytes that ensheath the conduit system. Data are representative of 3 different experiments (2 mice per experiment).
HEV pockets are plastic structures. (A) The right panel in the diagram represents a situation in which lymphocytes are not engulfed within pockets but simply migrating between 2 ECs while the left panel represents lymphocytes present in a true structured pocket underneath the HEV. L = Lymphocyte. (B) Confocal image of a LN section from a ubiquitin-GFP chimera (green) stained for ICAM-1 (red) and sytox 63/nuclei (blue) Arrowheads indicate the inner membrane of the EC directly in contact with lymphocytes. (C) Confocal image of a LN section from a ubiquitin-GFP chimera (green) stained for ERTR-7 (red), Desmin (white) and sytox 63/nuclei (blue). Arrows indicate the membranes of myofibroblasts/pericytes that ensheath the conduit system. Data are representative of 3 different experiments (2 mice per experiment).
HEVs pockets dynamically modulate HEV shape
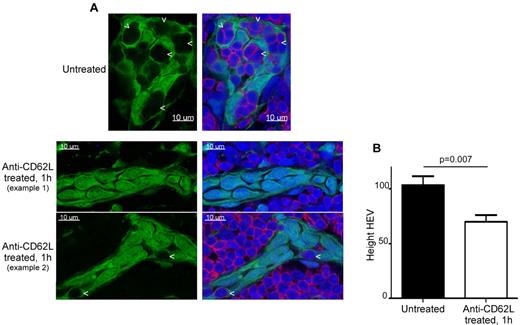
HEVs are characterized by cuboidal cells as opposed to the typical flat endothelial cells found in regular venules.13,14 To determine whether this shape is an inherent property of the HEV EC themselves or if it arises as a result of the presence of lymphocytes in these pockets, we treated chimeric mice with an anti-CD62L blocking antibody for 1 hour (Figure 3). Under these conditions, lymphocyte rolling on HEVs—and hence LN homing—is blocked.16 On such treatment, lymphocyte pockets in HEVs disappeared and HEVs concomitantly flattened (Figure 3A). Morphometric analysis demonstrated that the height of LN HEV decreased on anti-CD62L treatment, representing about 68% of the height of untreated HEVs (Figure 3B). Therefore, these data suggest that the typical cobblestone shape of HEV ECs is not intrinsic but rather results from the continuous and transient accumulation of lymphocytes within these pockets. These observations also demonstrate that these holding sites are not preformed, rigid hollow caverns within which lymphocytes accumulate, but rather spaces created by the accumulation of trafficking lymphocytes that have not yet passed into the perivascular channel (PVC) on their way into the LN parenchyma. In agreement with this concept, we never observed HEVs in which pockets were free of lymphocytes.
Blockade of lymphocyte homing to LN induces the disappearance of HEV pockets. (A) Confocal images of LNs section from a RAG-2°/°ubiquitin-GFP chimera (green) injected or not intravenously for 1 hour with 100 μg of anti-CD62L blocking Ab (MEL-14) and stained for CD3 (red) and sytox 63/nuclei (blue). Arrowheads point to HEV pockets. (B) Height (and standard error) in arbitrary units of LN HEV (see “Morphoretic analysis of HEV”). Data are representative of 2 different experiments (2 mice per experiment, ∼ 15 analyzed HEVs for each condition per experiment).
Blockade of lymphocyte homing to LN induces the disappearance of HEV pockets. (A) Confocal images of LNs section from a RAG-2°/°ubiquitin-GFP chimera (green) injected or not intravenously for 1 hour with 100 μg of anti-CD62L blocking Ab (MEL-14) and stained for CD3 (red) and sytox 63/nuclei (blue). Arrowheads point to HEV pockets. (B) Height (and standard error) in arbitrary units of LN HEV (see “Morphoretic analysis of HEV”). Data are representative of 2 different experiments (2 mice per experiment, ∼ 15 analyzed HEVs for each condition per experiment).
If HEV pockets are not rigid but adaptable structures, we reasoned that they should be highly dynamic structures continuously altered in size and location by lymphocyte migration from blood to LN parenchyma. To appreciate such dynamics, we irradiated RAG2°/° ubiquitin-GFP mice and reconstituted them with actin-CFP bone marrow cells, allowing us to image the immigration of endogenous CFP+ lymphocytes through the GFP+ HEVs. Reconstituted mice were anesthetized and their popliteal LN surgically exposed for 2-Photon (2P) imaging. Because the HEV EC membranes forming these pockets are ∼ 100 nm thick (Figure 1C) and highly motile in X, Y, and Z directions, dynamic imaging of entire HEV pockets was not possible. Therefore, we examined highly resolved single “slices” of shallow HEVs to appreciate the overall dynamics of HEV ECs. In these videos, the continuous ballet of CFP+ cells entering the GFP+ HEVs revealed that HEV pockets are highly dynamic structures moving in many directions as a result of lymphocyte displacement (supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
HEVs adapt lymphocyte entry to lymphocyte egress in the LN
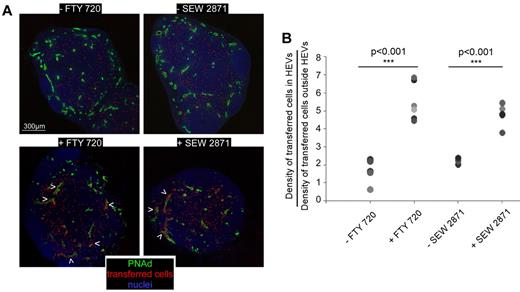
If HEVs are the site of adjustment of lymphocyte entry into the LN to maintain constant cell number in the face of ongoing egress, one would expect them to limit LN access to blood-circulating lymphocytes if the cellularity of this LN is maximal, whereas when the population of a resting LN decreases as a result of lymphocytes egress, HEVs should immediately counterbalance this cellular loss by allowing the entrance of blood circulating lymphocytes. The capacity of HEV EC to generate transient collections of trafficking lymphocytes would provide an elegant solution to this adjustment requirement—lymphocytes could be “stored” within the EC, being held there when the LN was “full” and released if the population declined as lymphocytes exited the LNs. Such a system makes sense in LNs that are densely packed organs with an only slowly changeable volume that imposes space limitations. If this concept is correct, blood circulating lymphocytes should less efficiently enter the parenchyma of a LN in which lymphocyte egress is blocked and in which cellularity would be maintained at a maximum. As a first attempt to test this hypothesis, we treated wild type (WT) mice with FTY-720 and SEW-2871, 2 drugs known to interfere with the sphingosine-1-phosphate (S1P) pathway involved in lymphocytes egress from lymphoid organs.17,18 Such treatment induces the sequestration of lymphocytes in SLOs and leads to a concomitant lymphopenia (supplemental Figure 1A). Drug-treated and control animals were injected intravenously with WT labeled lymphocytes and euthanized 1 hour later. The LNs were sectioned, stained for PNAd expression, and the ratio of labeled lymphocyte densities in HEVs and LN parenchyma calculated for each condition. The results showed that many more of the transferred lymphocytes in FTY-720 and SEW-2871 treated mice remained in the HEVs during the homing assay compared with control animals, indicating a reduced capacity to access the LN parenchyma (Figure 4A-B and supplemental Figure 1C). These results, while consistent with the general expectations of our model, are not black and white. However, drug-induced lymphopenia is never total and some cells still egress the LNs of treated mice, potentially allowing the entry of the same number of cells according to our hypothesis. In a control animal, the cohort of labeled cells injected in the mouse represents a small percentage of the endogenous blood-circulating lymphocyte population. Therefore, many more endogenous “black” cells than labeled transferred cells enter the untreated LNs during the 1 hour homing assay. On the contrary, in drug-treated animals, the great majority of endogenous cells is sequestered within SLOs and therefore no longer circulates in the blood (supplemental Figure 1A). In addition, a higher fraction of blood circulating T cells is composed of effector/memory CD62Llo cells that no longer compete with the transferred cells in the LN homing assay (supplemental Figure 1B). As a consequence, if the HEVs of control and treated animals were supporting the same influx of cells, many more transferred cells should have accessed the LN parenchyma of treated mice during the 1 hour homing assay. Despite this, we observed that lymphocytes transferred in drug-treated mice were delayed in their capacity to access the LN parenchyma.
S1P1 blockade limits blood lymphocyte access to the LN parenchyma. WT mice were either treated or not with FTY-720 (36 hours) or SEW-2871 (8 hours) and injected intravenously with 10 millions of CMTMR labeled lymphocytes (red). (A) One hour later, LNs were harvested, sectioned in multiple slices, stained for PNAd (green) and Sytox63/nuclei (blue) and analyzed by confocal microscopy. The position of each individual cell is highlighted with a red dot. Arrowheads indicate HEVs in which transferred cells accumulate. (B) For each LN, densities of transferred cells inside and outside PNAd+ HEVs were calculated and presented as a ratio. Each symbol represents the averaged ratio calculated from all the imaged slices of a given LN. Data are representative of 3 different experiments (2 or 3 mice per experiment, minimum of 3 LNs per mouse).
S1P1 blockade limits blood lymphocyte access to the LN parenchyma. WT mice were either treated or not with FTY-720 (36 hours) or SEW-2871 (8 hours) and injected intravenously with 10 millions of CMTMR labeled lymphocytes (red). (A) One hour later, LNs were harvested, sectioned in multiple slices, stained for PNAd (green) and Sytox63/nuclei (blue) and analyzed by confocal microscopy. The position of each individual cell is highlighted with a red dot. Arrowheads indicate HEVs in which transferred cells accumulate. (B) For each LN, densities of transferred cells inside and outside PNAd+ HEVs were calculated and presented as a ratio. Each symbol represents the averaged ratio calculated from all the imaged slices of a given LN. Data are representative of 3 different experiments (2 or 3 mice per experiment, minimum of 3 LNs per mouse).
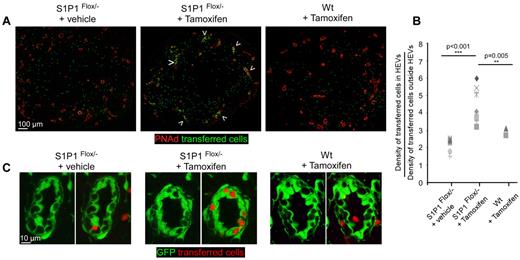
Because ECs express many S1P receptors, including S1P1, these drugs are not perfect tools for examining the issue of cellular trafficking.19 Unfortunately, S1P1°/° mice are not viable and using their fetal livers as a source of progenitors fails to generate chimeric mice with normal levels of lymphocytes.17,20 We therefore generated a mouse in which S1P1 deletion can be conditionally induced just in lymphocytes. Ubiquitin-Cre ESR1 mice were crossed to S1P1flox/− mice. In the former mice, the cre enzyme is released in the nucleus of all cells on tamoxifen injection while in the latter, all cells possess a functional S1P1 allele flanked by 2 lox P sites while the other allele is not functional. In these double transgenic mice, all cells are S1P1 sufficient but are expected to genetically lose S1P1 expression on tamoxifen injection. Because we wanted to generate mice in which only lymphocytes would show loss of S1P1 expression, we irradiated RAG 2°/° ubiquitin-GFP mice and reconstituted them with either WT bone marrow or a mixture of bone marrow cells isolated from RAG 2°/°WT (80%) and ubiquitin-Cre EsR1-S1P1flox/− (20%). These mice will be referred to as S1P1flox/− chimeras. In these chimeric mice, all cells should be S1P1 sufficient before tamoxifen injection but only lymphocytes (and 20% of the remaining hematopoietic cells) would become S1P1 deficient on tamoxifen injection. Reconstituted chimeras were treated or not with tamoxifen and 1 week later, their lymphopenia was evaluated as a result of lymphocytes sequestration in SLOs (supplemental Figure 2). In tamoxifen-treated S1P1flox/− chimeras, we observed a 70% reduction of blood lymphocytes, indicating that S1P1 deficiency on lymphocytes is sufficient to induce their sequestration in LNs. We then adoptively transferred a cohort of labeled WT lymphocytes in the various groups of mice and after 1 hour stained LNs sections for PNAd expression (Figure 5A-B). The data show that lymphocytes accessed the LN parenchyma of untreated S1P1flox/− chimeras and WT chimeras treated with tamoxifen equally. In contrast, in tamoxifen-treated S1P1flox/− chimeras, we observed that the lymphocytes were impaired in this process, indicating that lymphocyte sequestration in LNs controls the influx of blood circulating lymphocytes. We then focused our attention on the GFP+ PNAd+ HEVs of these different groups of mice, wishing to investigate if the lymphocytes were specifically held in the LN HEV pockets of S1P1flox/− chimeras treated with tamoxifen. As presented in Figure 5A and C, most transferred lymphocytes had already left the HEVs of controlled LNs during the 1 hour homing assay. On the contrary, HEVs of the tamoxifen-treated S1P1flox/− chimera contained numerous labeled lymphocytes confined to these pockets, indicating that these structures that only transiently hold lymphocytes in a normal situation were still occupied by lymphocytes in overloaded LNs.
Lymphocytes sequestration in LN limits blood lymphocytes access to the LN parenchyma. RAG-2°/°ubiquitin-GFP chimera were irradiated and reconstituted with either WT bone marrow cells or a mixture of bone marrow cells isolated from RAG-2°/°WT mice (20%) and ubiquitin-Cre Esr1 × S1P1flox/− mice (80%). Reconstituted chimeras were treated or not with tamoxifen and injected with 10 millions of CMTMR labeled lymphocytes (green). One hour later, LNs were harvested, sectioned, stained for PNAd (red) and analyzed by confocal microscopy (A). Arrowheads indicate HEVs in which many transferred cells accumulate. (B) Densities of transferred cells inside and outside PNAd+ HEVs were calculated for each analyzed section and presented as a ratio. (C) A typical HEV imaged in control and tamoxifen-treated ubiquitin-Cre Esr1 × S1P1flox/− is shown. Data are representative of 3 different experiments (2 or 3 mice per experiment, minimum of 3 LNs per mouse).
Lymphocytes sequestration in LN limits blood lymphocytes access to the LN parenchyma. RAG-2°/°ubiquitin-GFP chimera were irradiated and reconstituted with either WT bone marrow cells or a mixture of bone marrow cells isolated from RAG-2°/°WT mice (20%) and ubiquitin-Cre Esr1 × S1P1flox/− mice (80%). Reconstituted chimeras were treated or not with tamoxifen and injected with 10 millions of CMTMR labeled lymphocytes (green). One hour later, LNs were harvested, sectioned, stained for PNAd (red) and analyzed by confocal microscopy (A). Arrowheads indicate HEVs in which many transferred cells accumulate. (B) Densities of transferred cells inside and outside PNAd+ HEVs were calculated for each analyzed section and presented as a ratio. (C) A typical HEV imaged in control and tamoxifen-treated ubiquitin-Cre Esr1 × S1P1flox/− is shown. Data are representative of 3 different experiments (2 or 3 mice per experiment, minimum of 3 LNs per mouse).
Discussion
Overall, these data suggest that HEVs are more than passive transfer points for lymphocytes from blood to LN parenchyma. By accommodating lymphocytes in pockets where they can be held outside of the PVC until needed to replace exiting lymphocytes, HEVs behave as traffic control checkpoints that flexibly adapt to the needs of the resting LN as it seeks to balance lymphocyte entry and exit rates. This in turn allows the LN to constantly function at its maximal capacity at steady state.
Given the extreme cellular density of a LN, one could wonder how the space freed by cells leaving in the medulla would immediately be available to cells present in HEVs located hundreds microns away. Importantly, lymphocytes do not physically exit the parenchyma from LN medullary sinuses but from thinner cortical sinuses located nearby HEVs.21-23 Therefore, the juxtaposition of entry and exit sites would confine the regulation of entry/exit fluxes to very restricted regions of LNs. By itself, this juxtaposition is not sufficient to explain how the cellular density of the parenchyma is constantly sensed by the ECs and/or the nested lymphocytes. However, when lymphocytes exit HEV pockets, they enter the PVC and adapt a typical elongated shape likely imposed by the small diameter of the PVC.24 Lymphocytes further progress into this narrow corridor until they find an exit point (flap) formed by the membranes of 2 adjacent FRCs through which they squeeze out in the parenchyma.12 In a fully loaded LN, the cellular pressure in the parenchyma may narrow the diameter of the PVC and concomitantly increase the resistance of the exit flaps. In this situation, the lymphocytes would be retained for a longer period of time in the pockets until lymphocytes exit in the cortical sinuses and release some pressure on the flaps and the PVC.
While hypothetical, this model resolves another logistical issue. When a few lymphocytes exit the parenchyma via a given cortical sinus, our model suggests that the nearby HEVs should counterbalance this cellular loss. As an HEV is formed by hundreds of ECs able to retain lymphocytes, how can each of these ECs know the number of cells they should individually release from their pockets? As ECs constantly replace the lymphocyte content of the underlying PVC, it is likely that any lymphocyte exiting the PVC would be immediately replaced by lymphocytes nested in the closest EC sitting on top of this portion of the PVC, thus linking local pressure relief to trafficking and flux through the HEV pockets.
One question that arises from our observations is how lymphocytes nested in HEV pockets exit from these structures to access the PVC. To date, the most important directional cues known to attract and retain naive lymphocytes are chemokines such as CCL21 or CXCL13.25-29 Interestingly, in 1976, Anderson et al observed that on a subcutaneous injection of horseradish peroxidase, this small tracer “crossed the reticular sheath, penetrated spaces between endothelial cells where it surrounded emigrating lymphocytes, and then entered the venular lumen.”13 In 2000, the same group described the function of the conduit system and demonstrated that this network of reticular fibers was involved in the transport of lymph-borne chemokines to the abluminal and luminal surfaces of HEVs.30 Altogether, these results suggest that lymph-borne chemokines are transported from the conduit system to the basal membrane of the HEV before their presentation on the luminal side of the HEV, possibly creating a local gradient of chemokines that lymphocytes nested in the HEV pockets may sense to find the exit site toward the PVC.
One question that still animates numerous debates in the field is the exact mode of lymphocyte migration across HEV ECs. While some of these studies suggested that lymphocytes migrate between 2 adjacent ECs (paracellular migration),13,31,32 others have claimed that lymphocytes migrate within the EC (transcellular migration), possibly via the formation of transendothelial channels.33-36 Our data do not support or reject any of these models and the precise cascade of events leading to the entry of lymphocytes into the HEV pockets remains to be definitely determined.
In summary, our data provide new insight into how HEVs adjust lymphocyte entry fluxes to balance lymphocyte exit fluxes in noninflamed LNs. Such control may of course be switched off or overridden during an active immune response. After inflammation, HEVs located in the draining LN increase their capacity to recruit naive lymphocytes and, at the same moment, lymphocytes egress is transiently blocked, a phenomenon known as the “LN shut down.”37,38 Instead of creating a bottleneck situation as one would expect from a simple application of our pressure-gated access model, this combination of events induces a tremendous LN swelling because of a massive increase in total cell numbers, despite the presumed structural limitation of the surrounding capsule. Given the results presented here, we suggest that there must be mechanisms that relieve the physical constraint of pressure build-up because of excess cell entry and subsequent proliferation (eg, tension release within the structure of the capsule) and/or change the gating behavior we have described, mechanisms whose unraveling should prove valuable for better understanding lymphoid tissue function in health and disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank B. Bréart, S. Schwab, and R. Proia for providing the bone marrow cells of Ubi-cre ESR-1 × S1P1flox/− mice and constructive comments on the manuscript.
This research was supported in part by CNRS, Inserm, the Agence Nationale de la Recherche, the Association pour la Recherche sur le Cancer, the Institut National du Cancer, and the Intramural Research Program of NIAID, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.L.S., C.M., R.N.G. and M.B. designed research; S.L.S., C.M., I.M., A.J. and J.P.L. performed the experiments; and R.N.G. and M.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Bajénoff, Centre d'Immunologie de Marseille Luminy, Parc Scientifique et Technologique de Marseille Luminy, Case 906, 13288 Marseille, France; e-mail: bajenoff@ciml.univ-mrs.fr.
References
Author notes
C.M. and S.L.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal