Abstract
For patients with hematologic malignancies at high risk of relapse who do not have matched donors, a suitable alternative stem cell source is the HLAhaploidentical 2 or 3-loci mismatched family donor who is readily available for nearly all patients. Transplantation across the major HLA barrier is associated with strong T-cell alloreactions, which were originally manifested as a high incidence of severe GVHD and graft rejection. The present review shows how these obstacles to successful transplantation were overcome in the last 15 years, making full haplotype-mismatched transplantation a clinical reality that provides similar outcomes to transplantation from matched unrelated donors. The review also discusses the advantages and drawbacks of current options for full haplotypemismatched transplantation and highlights innovative approaches for re-building immunity after transplantation and improving survival.
Introduction
Despite advances in chemotherapy, allogeneic hematopoietic stem cell transplantation (HSCT) remains the best postremission therapy for patients with acute leukemia and unfavorable prognostic features at diagnosis, primary induction failure, or in second and third complete remission (CR). Even though HLA-identical siblings are the ideal source of hematopoietic stem cells (HSCs), only 25% of patients have such donors. Therefore, an alternative HSC source is needed for majority of patients. Alternative options include matched unrelated donors (MUDs), unrelated donor umbilical cord blood (UD-UCB), and full haplotype-mismatched related donor.
Each alternative source has its own particular advantages and drawbacks. Suitable MUD are found for 60% to 80% of whites but only for 10% of ethnic minorities.1 Using molecular probes improves tissue typing and lessens the risk of GVHD by very accurately matching unrelated donor-recipient pairs but reduces the probability of finding a suitable donor. Furthermore, as months can pass while identifying the donor and harvesting the HSCs, many acute leukemia patients relapse while awaiting transplantation.
UD-UCB offers the advantages of a shorter search time than MUD and acceptance of 2- of 6-antigen mismatch. In adults, however, the great discrepancy between body weight and the number of HSC in a standard cord blood unit, particularly if a 2-antigen mismatch is involved, delays hematopoietic reconstitution and increases the risk of graft failure. This drawback was partially overcome using double cord blood units.2
The advantages of transplantation from a full haplotype-mismatched family member include: availability for almost all patients, choice of best donor from a panel of candidate family members, no undue delay in obtaining the graft and finally, easy access to donor-derived cellular therapies if required after transplantation.
Major drawbacks are the very strong graft-versus-host and host-versus-graft alloresponses because of the high frequency of T cells that recognize major class I or II HLA disparities between donor and recipient.3 Consequently, early attempts to use full haplotype-mismatched donors in leukemia patients were not successful. Although ex-vivo T cell depletion of mismatched bone marrow with soybean agglutination and E-rosetting to reach the target of 2 to 4 × 104 T cells/kg recipient body weight prevented GVHD in children with severe combined immunodeficiency,4 30% of HLA-haploidentical grafts were rejected by leukemia patients because of anti–donor cytotoxic T lymphocyte precursors (CTLp) that survived supra-lethal conditioning.5,6
From the 1980s onwards, attempts to overcome the HLA barrier focused on strengthening myeloablation and immunosuppression in the conditioning regimen7,8 or on partially depleting the graft of T cells using anti–thymocyte globulin (ATG) or the T10B9 monoclonal antibody in conjunction with in vivo immunotoxin,9 and with posttransplantation cyclosporine and steroids. A major advance came with a graft containing a mega-dose (on average > 10 × 106 CD34+ cells/kg body weight) of extensively T cell–depleted G-CSF-mobilized, peripheral blood hematopoietic progenitor cells, following a myeloablative conditioning regimen based on total body irradiation (TBI). This strategy ensured for the first time a high engraftment rate across the HLA barrier in the absence of GVHD.
In recent years, interest in T cell–replete full haplotype-mismatched HSCT was reawakened by new transplant strategies for GVHD prophylaxis, such as G-CSF–primed grafts, posttransplantation rapamycin, or high-dose cyclophosphamide in combination with other immunosuppressive agents.
Each of these 2 current options for full haplotype-mismatched HSCT presents an intrinsic challenge. In T cell–depleted HSCT, the minimal residual T lymphocytes in the grafts successfully prevent lethal GVHD without any posttransplantation immunosuppression, but at the same time the small number of T cells infused leads to delayed immune reconstitution. Thus, the present challenge is to accelerate immune reconstitution by adoptive transfer of nonalloreactive pathogen-specific or broad repertoire T lymphocytes, depleted of alloreactive clones.
T cell–replete HSCT presents 2 major challenges. Although the high T-cell content of the graft potentially enhances the graft-versus-leukemia (GVL) effect, the same T cells induce significant GVHD-related morbidity and mortality. However, when using other strategies to prevent GVHD in T cell–replete HSCT, such as administration of high-dose cyclophosphamide after transplantation to prevent GVHD (performed mostly following reduced intensity protocols), a high incidence of leukemia relapse has emerged as a major problem. Therefore, efforts are being made to reduce the incidence and severity of GVHD and to prevent leukemia relapse.
This review outlines and evaluates these opposing modalities and discusses future directions in full haplotype-mismatched HSCT.
T cell–replete full haplotype-mismatched HSCT
G-CSF–primed graft
According to Huang et al in Beijing, G-CSF–primed donor HSCs and robust posttransplantation GVHD prophylaxis reduced the risk of transplantation-related mortality (TRM) and improved long-term survival.10 A follow-up study of 250 mismatched transplants in acute leukemia patients (89 at high-risk) reported nearly 100% full-donor engraftment, with 45.8% and 13.4% cumulative incidence of grade 2 to 4 and 3 or 4 acute GVHD (aGVHD), respectively. The cumulative incidence of chronic GVHD (cGVHD) was 53.9% (extensive in 22.6%). The 3-year probability of disease-free survival (DFS) was 70.7% and 55.9% in standard and high-risk acute myeloid leukemia (AML) patients, and 59.7% and 24.8%, respectively, in acute lymphoblastic leukemia (ALL).11 Interestingly, survival in patients with acute leukemia was higher in haploidentical recipients than in matched sibling recipients (42% vs 20%, P = .048), presumably because of a stronger GVL effect.12
The drop in the incidence and severity of GVHD deserves further investigation in Western populations.13 Likewise, the mechanism of GVHD modulation, suggested by the authors to be mediated by G-CSF priming of T cells in the bone marrow, is still obscure. Indeed, ATG in the conditioning and the powerful posttransplantation GVHD prophylaxis probably provided a major contribution to controlling alloreactivity.
Rapamycin-based GVHD prophylaxis
Because in vitro evidence showed that rapamycin did not affect T regulatory (Treg) cells, Peccatori et al14 developed a protocol with rapamycin and mycophenolate mofetil (MMF) for GVHD prophylaxis after conditioning with treosulfan, fludarabine, ATG, and a single dose of rituximab to treat 39 AML and 9 ALL advanced-stage patients (median age, 50 years). The cumulative incidence of aGVHD was 29% for grade 2 to 4 and 13% for grade 3 or 4. Twelve of 59 patients developed cGVHD. TRM and relapse were 25% and 44%, respectively, and projected 1-year overall survival was 43%. Early immune reconstitution polarized toward central memory cells, with significantly higher peripheral blood Treg (CD4+CD25+CD127−Foxp3+) counts than in donors.
Because rapamycin has never before been used in the clinical setting of full haplotype-mismatched transplantation, this approach deserves particular attention as it explores the rapamycin effects on Treg induction and GVHD prevention. However, the short follow-up precludes definitive conclusions.
High-dose cyclophosphamide-based GVHD prophylaxis
In the 1970s, Owens and Santos demonstrated in rodents that a short course of high-dose cyclophosphamide soon after bone marrow transplantation (BMT) targeted activated donor or host alloreactive T cells.15 This approach was abandoned when a randomized clinical trial demonstrated that a lower dose of cyclophosphamide was less efficacious than cyclosporine as prophylaxis against aGVHD after HLA-matched sibling alloBMT.16 However, the observation that cyclophosphamide is nontoxic to HSCs because of their high expression of the detoxifying enzyme aldehyde dehydrogenase,17 and the demonstration of Prigozhina group that high-dose cyclophosphamide administration can reduce GVHD and graft rejection in mice, without adverse effects on stem cell engraftment,18 led to renewed clinical attempts using this approach.
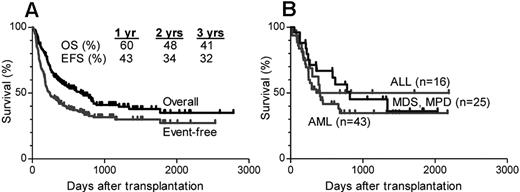
Clinical trials by the Johns Hopkins and Fred Hutchinson Cancer Research Center groups evaluated a nonmyeloablative protocol of cyclophosphamide, fludarabine, and 2 Gy TBI and posttransplantation GVHD prophylaxis with cyclophosphamide (50 mg/kg days 3 and 4), MMF (days 5-35), and tacrolimus (days 5-180).19 Engraftment was sustained in 87% of 210 acute leukemia patients treated by the Johns Hopkins group. Grade 2 to 4 aGVHD occurred in 27% of patients, and grade 3 or 4 occurred in 5% and cGVHD in 15%. The cumulative incidences of relapse and nonrelapse mortality were 55% and 18%, respectively. A total of 113 patients died of relapse (79), infections (15), pulmonary complications (7), GVHD (5), or other causes (7). Three-year overall survival and event-free survival (EFS) were 41% and 32%, respectively20 (Figure 1).
Nonmyeloablative conditioning after transplantation with high-dose cyclophosphamide. The figure summarizes the results of 3 similar clinical trials of nonmyeloablative conditioning and transplantation of partially HLA-mismatched bone marrow at Johns Hopkins, Fred Hutchinson Cancer Research Center, or BMT Group of Georgia and Hahnemann University Hospital. Reprinted from Munchel et al with permission.97 (A) Actuarial curves of overall survival (OS) and EFS in 210 patients undergoing nonmyeloablative HSCT with posttransplantation cyclophosphamide. (B) OS in patients with ALL, AML, or myelodysplastic syndrome (MDS) or myeloproliferative disorder (MPD).
Nonmyeloablative conditioning after transplantation with high-dose cyclophosphamide. The figure summarizes the results of 3 similar clinical trials of nonmyeloablative conditioning and transplantation of partially HLA-mismatched bone marrow at Johns Hopkins, Fred Hutchinson Cancer Research Center, or BMT Group of Georgia and Hahnemann University Hospital. Reprinted from Munchel et al with permission.97 (A) Actuarial curves of overall survival (OS) and EFS in 210 patients undergoing nonmyeloablative HSCT with posttransplantation cyclophosphamide. (B) OS in patients with ALL, AML, or myelodysplastic syndrome (MDS) or myeloproliferative disorder (MPD).
Thus, the high relapse rate, which was probably the result of poor disease debulking by the nonmyeloablative conditioning and lack of GVHD-related GVL effect, dampened the advantage of a relatively low TRM.20
Taken together, T cell–replete full haplotype-mismatched HSCT has developed in recent years and become a leading alternative option in several centers. However, higher rates and severity of aGVHD and cGVHD can significantly impair the quality of life of disease survivors and could lead to higher relapse rates when applied after reduced intensity conditioning (RIC) or when using aggressive immune suppression.
T cell–depleted full haplotype-mismatched HSCT
Mega-dose HSCT after myeloablative TBI-based conditioning
After myeloablative conditioning regimen, a high dose (≥ 10 × 106/kg) of purified CD34+ cells promotes engraftment in the majority of acute leukemia patients.
The first clinical trial was based on preclinical studies that showed that full-donor engraftment without GVHD could be achieved by escalating doses of T cell–depleted BM in a stringent mouse model for T cell–mediated BM allograft rejection21 or in sublethally irradiated mice.22 High-risk patients, most with acute leukemia, received BM and G-CSF–mobilized peripheral blood progenitor cells, which were depleted of T cells by soybean agglutination and E-rosetting. The conditioning included TBI (8 Gy in single fraction), thiotepa (10 mg/kg), cyclophosphamide (100 mg/kg), and rabbit ATG (25 mg/kg). A high engraftment rate (16 of 17) was achieved, with 18% incidence of aGVHD, even though no posttransplantation immunosuppression was administered.23,24
In subsequent reports, several modifications were made: fludarabine replaced cyclophosphamide in the conditioning protocol to reduce extra-hematologic toxicity, graft processing was improved by positive immunoselection of CD34+ cells,25,26 and posttransplantation G-CSF administration was stopped after it was observed to impair IL-12 production by dendritic cells, leading to abnormal antigen presentation and T-cell activation.27
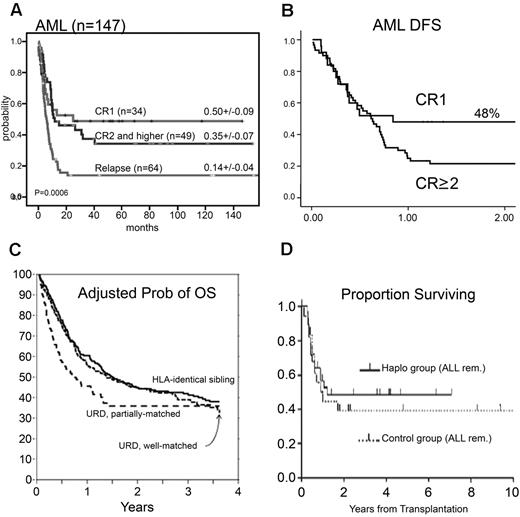
These strategies ensured primary sustained full donor type engraftment in 95% of 255 acute leukemia patients with an extremely low incidence of aGVHD and cGVHD.28 The relapse rate was 18% in AML and 30% in ALL patients who were transplanted in any CR. The cumulative-incidence of TRM was 0.36 (95% confidence interval [CI], 0.29-0.53) for 145 patients in any CR at transplantation, rising to 0.58 (95% CI, 0.40-0.65) for 110 patients transplanted in relapse.28 With a maximum follow-up of 17 years, leukemia-free survival (LFS) was, respectively, 43% and 30% in AML and ALL patients in any CR (Figure 2A). Although patients who were transplanted in chemoresistant relapse had poor outcomes, an 18% LFS for advanced AML patients is worth noting. All long-term survivors enjoy an excellent quality of life with no cGVHD.29
Representative results of haploidentical studies. (A) Results from Perugia describing long-term EFS probability of AML patients transplanted in either remission or relapse. Reprinted from Aversa et al with permission.28 (B) Results from the European Group for Blood and Marrow Transplantation survey of 173 high-risk adult leukemia patients undergoing fully haploidentical transplantation in 49 centers. The figure describes LFS of AML patients according to disease status at time of transplantation (25 in CR1 and 148 in CR ≥ 2). Reprinted from Ciceri et al with permission.30 (C) An observational study based on data from the statistical center of the Center for International Blood and Marrow Transplant Research, summarizing the results of 584 patients in 151 center undergoing allogeneic transplantation for AML in first remission and unfavorable cytogenetics at diagnosis. The figure describes adjusted overall survival probability based on stem cell origin. Reprinted from Gupta et al with permission.98 As can be seen, the results presented are comparable and less favorable than that described for AML patients undergoing haploidentical hematopoietic transplantation in first CR. (D) The probability of DFS for ALL pediatric patients who received positive selected transplants from haploidentical donors (n = 28) or unmanipulated BMTs from matched unrelated donors (n = 18) while in remission. Reprinted from Lang et al with permission.32
Representative results of haploidentical studies. (A) Results from Perugia describing long-term EFS probability of AML patients transplanted in either remission or relapse. Reprinted from Aversa et al with permission.28 (B) Results from the European Group for Blood and Marrow Transplantation survey of 173 high-risk adult leukemia patients undergoing fully haploidentical transplantation in 49 centers. The figure describes LFS of AML patients according to disease status at time of transplantation (25 in CR1 and 148 in CR ≥ 2). Reprinted from Ciceri et al with permission.30 (C) An observational study based on data from the statistical center of the Center for International Blood and Marrow Transplant Research, summarizing the results of 584 patients in 151 center undergoing allogeneic transplantation for AML in first remission and unfavorable cytogenetics at diagnosis. The figure describes adjusted overall survival probability based on stem cell origin. Reprinted from Gupta et al with permission.98 As can be seen, the results presented are comparable and less favorable than that described for AML patients undergoing haploidentical hematopoietic transplantation in first CR. (D) The probability of DFS for ALL pediatric patients who received positive selected transplants from haploidentical donors (n = 28) or unmanipulated BMTs from matched unrelated donors (n = 18) while in remission. Reprinted from Lang et al with permission.32
An European Group for Blood and Marrow Transplantation retrospective study, analyzing the outcome of “mega-dose” HLA-haploidentical transplantation in several European centers, reported similar results with 48% EFS in AML patients in first CR30 (Figure 2B). These results are comparable with reported outcome in a large observational study, based on data from the statistical center of the Center for International Blood and Marrow Transplant Research, summarizing the results of 584 patients in 151 centers, undergoing allogeneic transplantation for AML in first remission with unfavorable cytogenetics at diagnosis (Figure 2C).
These results highlight several aspects that deserve closer attention:
High numbers of CD34+ cells can overcome the HLA barrier.
A threshold of 2 × 104 CD3+ T cells/kg almost completely prevents GVHD, even in the absence of any posttransplantation immunosuppression. It could be argued, however, that ATG in the conditioning protocol, with its prolonged plasma half-life, induced additional in vivo T-cell depletion of the graft, contributing to reduce GVHD incidence and severity.
A low posttransplantation leukemia relapse rate despite the lack of GVHD-related GVL effect. In our view, several factors contributed to these crucial data, including the myeloablative strength of the conditioning, donor-versus-recipient natural killer (NK) cell alloreactivity (see “Selecting good prognosis donors: NK-cell alloreactivity and the mother donor effect”) and absence of posttransplantation pharmacologic immunosuppression.
A relatively high TRM rate because of slow posttransplantation immune reconstitution, patients' clinical status and comorbidities, and disease stage at time of transplantation. Although most deaths were the result of opportunistic infections (CMV, invasive aspergillosis), the risk of life-threatening episodes slowly declined and reached a plateau after ∼ 1 year, confirming that complete immunologic reconstitution is achieved by this stage, in transplant recipients who do not receive immunosuppression and do not have any cGVHD.
Encouraging results were also demonstrated in pediatric patients. A mean dose of 20.7 × 106/kg purified CD34+, after myeloablative conditioning, which included OKT3, was administered to 39 children at the Tubingen University Hospital. Rapid primary engraftment was achieved in 36 with an extremely low incidence of GVHD. Relapse accounted for 13 deaths and TRM for 10. At a median follow-up of 2 years, 15 of 39 patients were alive and well.31 In a later study from the same group, the DFS of 28 patients with ALL/non-Hodgkin lymphoma in remission, who received positively selected haploidentical grafts, was comparable with that of a similar historical control group receiving unmanipulated MUD transplant (48% vs 38%; P = .6). Moreover, no significant difference was observed between the 2 groups in the 3-year probability of relapse32 (Figure 2D).
Similar results were also reported by Locatelli et al in Pavia.33 In contrast, a European multicenter analysis of 127 children with high-risk ALL reported a 5-year DFS of 27% for patients in CR. However, multivariate analysis detected a trend toward a center-related effect with better DFS in larger centers (39% vs 15%). An improved outcome with less relapse was also described for patients receiving a higher CD34+ cell dose, suggesting that a maximal CD34 dose should be infused whenever possible.34
Thus, a “mega-dose” of T cell–depleted full haplotype-mismatched HSCT successfully prevents grafts rejection as well as GVHD and results in survival rates similar to MUD transplants. Relatively high nonrelapse mortality rates were mostly the result of opportunistic infections. In the absence of GVHD and posttransplantation immunosuppression, there is a window for adoptive T-cell immunotherapy to improve immune reconstitution and outcomes.
How mega-dose transplants overcome the HLA barrier
Veto activity was defined in 1980 by Miller as the capacity to specifically suppress cytotoxic T-cell precursors directed against antigens presented by the veto cells.35 Over the years, different veto cell types, exerting their activity through distinct mechanisms, have been described. Thus, the term “veto” represents an operational definition and not a specific cell subpopulation. After the discovery that mega-dose transplants can overcome T cell–mediated rejection, Rachamim et al36 demonstrated in mixed lymphocyte reaction that cells within the CD34+ fraction specifically suppressed CTLp directed against their own antigens, but not against third-party antigens. This veto activity was shown to be mediated by TNF-α–induced deletion of recognizing effector T cells,37 and was also exhibited by ex vivo differentiated immature CD34+CD33+ and CD34−CD33+ myeloid cells.38 Likewise, immature dendritic cells, previously shown to induce immune tolerance, exhibited marked veto activity on CD8+ T cells through a perforin-based mechanism while suppressing CD4+ T cells through an MHC-independent mechanism mediated by the NO system.39 Thus, after transplantation of purified CD34+ cells, the likelihood of activation of anti–donor CTLp is proportional to the level of residual host T cells and is inversely correlated to the number of veto and other tolerizing cells. Veto activity could initially be exerted by infused CD34+ cells and their CD33+ progeny, as well as by CD11c+ immature dendritic cells.39 In addition, transplantation from HLA genotypes, which allow the rapid generation of alloreactive NK cells (see next section), can eradicate mature anti–donor CTLs escaping the veto cells. Thus, the mega-dose haploidentical CD34 inoculum is responsible to veto anti–donor CTLp and also to rapidly generate the second or third derivatives required to complete the eradication of host anti–donor T cells.
Selecting “good prognosis” donors: NK-cell alloreactivity and the “mother donor effect”
Seminal work by Ruggeri et al demonstrated that, in the setting of haploidentical hematopoietic transplantation, NK-cell alloreactivity is a powerful form of antileukemia immunotherapy.40
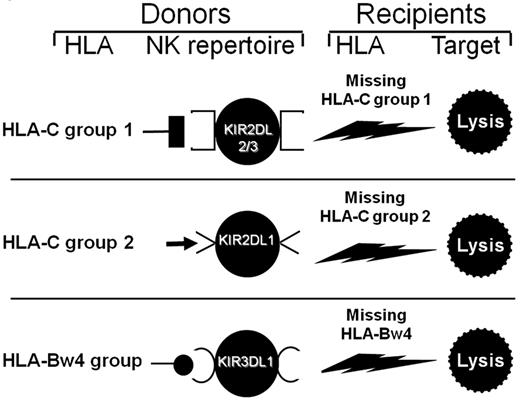
Human NK cells possess clonally distributed, inhibitory receptors termed “killer cell immunoglobulin-like receptors” (KIRs) that recognize allotypic determinants (KIR ligands) shared by certain HLA-class I allele groups (HLA-C group 2 alleles; HLA-C group 1 alleles; HLA-B alleles sharing the Bw4 specificity). Upon interaction with self-HLA KIR ligands, NK cells become “licensed/educated” to exert alloreactivity against allogeneic targets that do not express self-HLA KIR ligands. Thus, in full haplotype-mismatched HSCT, donor-versus-recipient NK-cell alloreactivity is effected by “licensed” NK cells of donor origin, which express as their only inhibitory receptor for self, a KIR whose ligand is absent on allogeneic targets. Therefore, they sense missing expression of the donor class-I KIR ligand on target cells and mediate alloreactivity (“missing self” recognition). Indeed, the engrafted stem cells give rise to an NK-cell repertoire of donor origin, which includes alloreactive clones that kill recipient leukemic cells. Evidently, donor NK cells mature in a bone marrow microenvironment where they are predominantly exposed to donor HLA (on hematopoietic cells), which shapes their repertoire to become fully functional and recipient-alloreactive.
Three KIR ligand-mismatches (in the graft-versus-host direction) trigger donor-versus-recipient NK-cell alloreactivity: (1) HLA-C1 present in donor/missing in recipient, (2) HLA-C2 present in donor/missing in recipient, and (3) HLA-Bw4 present in donor/missing in recipient (Figure 3).
Selecting donor/recipient pairs with donor-versus-recipient NK alloreactivity. All persons possess the KIR2DL2 and/or KIR2DL3 receptors for HLA-C group 1 alleles. If they have HLA-C group 1 allele(s) in their HLA type, they possess HLA-C1-specific NK cells, which are alloreactive against cells from persons who do not express HLA-C group 1 alleles (top panel). A total of 97% of persons possess the KIR2DL1 receptor for HLA-C group 2. If they possess HLA-C group 2 allele(s) in their HLA type, they have HLA-C2-specific NK cells, which mediate alloreactions against cells from persons who do not express HLA-C group 2 alleles (middle panel). In one study on a large cohort,41 functional analyses detected alloreactivity when NK clones were tested against HLA-C group mismatched allogeneic targets. Frequencies of alloreactive NK clones were high, that is, 8 ± 6 cells in 100 (mean ± SD) for HLA-C group 2 mismatches; 5 ± 3 cells in 100 for group 1 mismatches. Finally (bottom panel), 90% of persons possess the KIR3DL1 receptor for HLA-Bw4 alleles. When they have HLA-Bw4 allele(s) in their HLA type, they may have HLA-Bw4–specific NK cells that are alloreactive against Bw4-negative cells. When NK clones from HLA-Bw4–positive persons who possessed the KIR3DL1 gene were tested against allogeneic HLA-Bw4–negative targets, alloreactive NK clones were detected in 2 of 3 persons.41 Reprinted from Velardi (Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20:581-587) with permission.
Selecting donor/recipient pairs with donor-versus-recipient NK alloreactivity. All persons possess the KIR2DL2 and/or KIR2DL3 receptors for HLA-C group 1 alleles. If they have HLA-C group 1 allele(s) in their HLA type, they possess HLA-C1-specific NK cells, which are alloreactive against cells from persons who do not express HLA-C group 1 alleles (top panel). A total of 97% of persons possess the KIR2DL1 receptor for HLA-C group 2. If they possess HLA-C group 2 allele(s) in their HLA type, they have HLA-C2-specific NK cells, which mediate alloreactions against cells from persons who do not express HLA-C group 2 alleles (middle panel). In one study on a large cohort,41 functional analyses detected alloreactivity when NK clones were tested against HLA-C group mismatched allogeneic targets. Frequencies of alloreactive NK clones were high, that is, 8 ± 6 cells in 100 (mean ± SD) for HLA-C group 2 mismatches; 5 ± 3 cells in 100 for group 1 mismatches. Finally (bottom panel), 90% of persons possess the KIR3DL1 receptor for HLA-Bw4 alleles. When they have HLA-Bw4 allele(s) in their HLA type, they may have HLA-Bw4–specific NK cells that are alloreactive against Bw4-negative cells. When NK clones from HLA-Bw4–positive persons who possessed the KIR3DL1 gene were tested against allogeneic HLA-Bw4–negative targets, alloreactive NK clones were detected in 2 of 3 persons.41 Reprinted from Velardi (Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20:581-587) with permission.
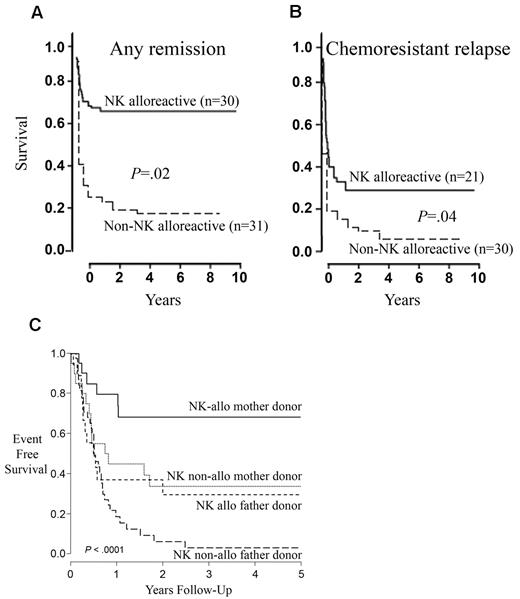
Donor-versus-recipient NK-cell alloreactivity improves outcomes of HLA haploidentical transplants by controlling leukemia relapse without causing GVHD. An updated analysis of 112 high-risk AML patients confirmed the presence of NK alloreactivity, reflected by significantly lower relapse rates and better EFS. Thus, in patients transplanted in CR, the cumulative incidence of relapse was significantly lower after transplantation from NK-alloreactive donors (3% vs 47%; P < .003) and the probability of survival was significantly improved with EFS of 67% in recipients of NK-alloreactive versus 18% non-alloreactive donors (P = .02; Figure 4A). A high 34% EFS rate was also observed in patients transplanted in relapse (Figure 4B).41 HSCT from haploidentical, NK-alloreactive donors was also reported to lower the risk of relapse in children with ALL.33,42,43
NK-cell alloreactivity and the “mother donor effect.” (A-B) Transplantation from haploidentical NK-alloreactive donors improves EFS. (A) EFS in patients transplanted in CR from NK-alloreactive versus non–NK-alloreactive donors. (B) EFS in patients transplanted in relapse from NK-alloreactive versus non–NK-alloreactive donors. Reprinted from Ruggeri et al with permission.41 (C) EFS of patients receiving parental donor haploidentical HSCT for acute leukemia stratified by both donor sex and NK alloreactivity (NK-alloreactive mother donor transplantation, N = 21; NK nonalloreactive mother donor transplantation, N = 20; NK-alloreactive father donor transplantation, N = 19; NK nonalloreactive father donor transplantation, N = 40). Reprinted from Stern et al with permission.44
NK-cell alloreactivity and the “mother donor effect.” (A-B) Transplantation from haploidentical NK-alloreactive donors improves EFS. (A) EFS in patients transplanted in CR from NK-alloreactive versus non–NK-alloreactive donors. (B) EFS in patients transplanted in relapse from NK-alloreactive versus non–NK-alloreactive donors. Reprinted from Ruggeri et al with permission.41 (C) EFS of patients receiving parental donor haploidentical HSCT for acute leukemia stratified by both donor sex and NK alloreactivity (NK-alloreactive mother donor transplantation, N = 21; NK nonalloreactive mother donor transplantation, N = 20; NK-alloreactive father donor transplantation, N = 19; NK nonalloreactive father donor transplantation, N = 40). Reprinted from Stern et al with permission.44
In addition to NK-cell alloreactivity, maternal donors (as opposed to any other donor-recipient family relationship) provided much better protection from leukemia relapse. The effect was independent of, and additive to, the beneficial effects of NK alloreactivity (Figure 4C).44 Better outcome of mother-to-child transplantation may be the result of maternal immune system exposure to fetal antigens during pregnancy and ensuing memory T-cell immunity against the child's paternal HLA haplotype.
T cell–depleted full haplotype-mismatched HSCT after RIC
Because high-intensity conditioning regimens are not feasible for elderly, heavily pretreated patients, or those with significant comorbidities, RIC regimens were investigated with diverse methods for achieving T-cell depletion.
CD3/CD19 depletion.
In Tubingen and Memphis, adults and children with acute leukemia were conditioned with fludarabine (150-200 mg/m2), thiotepa (10 mg/kg), melphalan (120 mg/m2), and OKT-3 (5 mg/day, day −5 to day 14). The graft, depleted of T lymphocytes by anti-CD3 and anti-CD19–conjugated magnetic beads, contained high numbers of NK cells and monocytes. Although 28 of 29 high-risk adult patients with refractory disease or relapse engrafted with full donor chimerism, the incidence of aGVHD (34% grade 2; 14% grade 3 or 4) was higher than in transplants with positively selected CD34+ cells. Overall survival was 31% with a median follow-up of 241 days. Relapse accounted for 12 of 20 deaths, infections for 7, and GVHD for 1.45
Very recently, Bader et al reported their experience with 22 children with acute leukemia, who were conditioned with fludarabine, melphalan, and thiotepa, and received a CD3/CD19-depleted graft.46 Patients engrafted rapidly, with 10.7% TRM and a 68% probability of survival at 3 years for those in remission at transplantation.46 Using the same protocol in pediatric ALL patients, González-Vicent et al recently showed significantly improved LFS, lower TRM, and lower aGVHD rates after haploidentical transplantation, compared to UD-UCB transplantation performed during the same period (41% ± 13% vs 26% ± 9%, 25% ± 9% vs 47% ± 9%, and 19% ± 7% vs 44% ± 10%, respectively).47
Although these data are very promising, the enhanced incidence of GVHD outcomes compared with that found after transplantation of positively selected CD34+ graft could be the result of the observed variability in the level of T-cell depletion and could be addressed by a more rigorous procedure currently developed by the Handgretinger group using magnetic beads coupled to anti-TCR αβ antibody.48
Alemtuzumab-based T-cell depletion.
A pilot study with 12 high-risk patients showed that including in vivo alemtuzumab in a myeloablative conditioning ensured full donor type engraftment of unmanipulated haploidentical HSCT, with a low 9% incidence of grade 2 to 4 aGVHD.49 In a later study, 49 high-risk patients (median age, 48 years) received a nonmyeloablative regimen (fludarabine, cyclophosphamide) and alemtuzumab for in vivo and in vitro T-cell depletion.50 Engraftment was successful in 94% of patients with 10.2% TRM and 8% severe GVHD.
Although high rates of engraftment with acceptable risk of GVHD and TRM were achieved, high relapse rates, probably because of a weak disease debulking by the nonmyeloablative protocol, markedly reduced overall survival to 31% at 1 year.
Table 1 summarizes some of the more common methodologies for achieving sustained engraftment of full haplotype-mismatched HSCT.
Common methodologies for achieving sustained engraftment of full haplotype mismatched HSCT
| Group/Reference . | Number . | Disease . | Method of TCD . | Conditioning . | GVHD prophylaxis . | Comment . |
|---|---|---|---|---|---|---|
| T-replete myeloablative | ||||||
| Beijing; 11 | 250 | 108 AML, 142 ALL | No TCD | G-CSF primed BM and PB, Ara-C, BU, Cy, simustine, ATG | CsA, MMF, MTX | Adult patients |
| Beijing; 99 | 42 | 24 ALL, 12 AML, 6 CML | No TCD | G-CSF primed BM and PB, Ara-C, BU, Cy, simustine, ATG | CsA, MMF, MTX | Pediatric patients |
| T-replete nonmyeloablative | ||||||
| Durham; 50 | 49 | 29 Leuk/MDS, 15 ML/MM, 2 CML, 3 MPD | No TCD | Alemtuzumab, Flu, Cy | MMF ± CsA | Adult patients |
| Toyama; 100 | 66 | 32 high-risk AL, 10 MDS, 19 Ly, 2 CML, 3 MM | No TCD | (2 Gy TBI or Flu) + ATG, BU, Mel | TAC ± mP | Adult patients |
| Baltimore; 101 | 185 | 106 lymphoid malignancy, 78 myeloid malignancies | No TCD | 2 Gy TBI, Cy, Flu | High-dose CY, TAC, MMF | Combined adults and pediatric patients |
| T-deplete myeloablative | ||||||
| Columbia, SC; 102 | 201 | 102 AML, 99 ALL | T10B9 or OKT3 | TBI, Ara-C, Cy, VP-16, ATG, mP | Partial TCD with OKT3 or T10B9 + mP, ATG, CsA | Combined adults and pediatric patients; 51% TRM at 5 years |
| Perugia; 26 | 104 | 67 AML, 37 ALL | CD34+ selection | 8 Gy TBI, Thio, Flu, ATG | None | Combined adults and pediatric patients; all high-risk patients |
| Multi-center; 30 | 266 | 173 AML, 93 ALL | CD34+ selection | TBI-based myeloablation in 74% of AML and 92% of ALL, ATG in majority | NA | All high-risk patients; better LFS for direct family member donor |
| Tubingen; 32 | 63 | 32 ALL, 13 AML/CML, 4 MDS, 4 Ly, 10 NM | CD34+ or CD133+ selection | BU or TBI + ATG/OKT3 + Cy + Thio or Cy + Flu | None | Pediatric patients |
| Bristol; 103 | 34 | 17 AML, 14 ALL, MDS/CML/biphenotypic AL 3 | Campath in the bag (7), CD34+ selection (27) | Cy + 14.4 Gy TBI + IV Campath or ATG | CsA in 7, none in 27 | Pediatric patients |
| Multi-center; 34 | 127 | Very high-risk ALL 102 in CR1-3 25 NR | Mostly CD34+ selection | Myeloablative, TBI in 76% | NA, 96% received ATG or ALG | Multicenter study on pediatric patients; improved LFS in larger centers |
| T-deplete nonmyeloablative | ||||||
| Tubingen; 45 | 29 | 16 AML, 7 ALL, 3 NHL, 2 MM, 1 CML | CD3/CD19 depletion | Flu, Thio, Mel, OKT-3 | None | Adults patients |
| Memphis; 104 | 22 | 9 AML, 9 ALL, 2 CML, 2 Ly | CD3 depletion | Flu, Thio, Mel, OKT-3 | MMF | Pediatric patients |
| Frankfurt; 46 | 59 | 15 ALL, 14 AML, 2 MDS, 13 RMS, 5 solid tumors, 10 NM | CD3/CD19 depletion | Flu, Thio, Mel | NA | Pediatric patients |
| Madrid; 47 | 29 | 18 ALL, 11 AML | CD3/CD19 depletion in 26; CD34+ selection in 3 | Flu, BU, Thio, mP | CsA alone (6) or ATG alone (8) or CsA + MTX (15) | Pediatric patients |
| Group/Reference . | Number . | Disease . | Method of TCD . | Conditioning . | GVHD prophylaxis . | Comment . |
|---|---|---|---|---|---|---|
| T-replete myeloablative | ||||||
| Beijing; 11 | 250 | 108 AML, 142 ALL | No TCD | G-CSF primed BM and PB, Ara-C, BU, Cy, simustine, ATG | CsA, MMF, MTX | Adult patients |
| Beijing; 99 | 42 | 24 ALL, 12 AML, 6 CML | No TCD | G-CSF primed BM and PB, Ara-C, BU, Cy, simustine, ATG | CsA, MMF, MTX | Pediatric patients |
| T-replete nonmyeloablative | ||||||
| Durham; 50 | 49 | 29 Leuk/MDS, 15 ML/MM, 2 CML, 3 MPD | No TCD | Alemtuzumab, Flu, Cy | MMF ± CsA | Adult patients |
| Toyama; 100 | 66 | 32 high-risk AL, 10 MDS, 19 Ly, 2 CML, 3 MM | No TCD | (2 Gy TBI or Flu) + ATG, BU, Mel | TAC ± mP | Adult patients |
| Baltimore; 101 | 185 | 106 lymphoid malignancy, 78 myeloid malignancies | No TCD | 2 Gy TBI, Cy, Flu | High-dose CY, TAC, MMF | Combined adults and pediatric patients |
| T-deplete myeloablative | ||||||
| Columbia, SC; 102 | 201 | 102 AML, 99 ALL | T10B9 or OKT3 | TBI, Ara-C, Cy, VP-16, ATG, mP | Partial TCD with OKT3 or T10B9 + mP, ATG, CsA | Combined adults and pediatric patients; 51% TRM at 5 years |
| Perugia; 26 | 104 | 67 AML, 37 ALL | CD34+ selection | 8 Gy TBI, Thio, Flu, ATG | None | Combined adults and pediatric patients; all high-risk patients |
| Multi-center; 30 | 266 | 173 AML, 93 ALL | CD34+ selection | TBI-based myeloablation in 74% of AML and 92% of ALL, ATG in majority | NA | All high-risk patients; better LFS for direct family member donor |
| Tubingen; 32 | 63 | 32 ALL, 13 AML/CML, 4 MDS, 4 Ly, 10 NM | CD34+ or CD133+ selection | BU or TBI + ATG/OKT3 + Cy + Thio or Cy + Flu | None | Pediatric patients |
| Bristol; 103 | 34 | 17 AML, 14 ALL, MDS/CML/biphenotypic AL 3 | Campath in the bag (7), CD34+ selection (27) | Cy + 14.4 Gy TBI + IV Campath or ATG | CsA in 7, none in 27 | Pediatric patients |
| Multi-center; 34 | 127 | Very high-risk ALL 102 in CR1-3 25 NR | Mostly CD34+ selection | Myeloablative, TBI in 76% | NA, 96% received ATG or ALG | Multicenter study on pediatric patients; improved LFS in larger centers |
| T-deplete nonmyeloablative | ||||||
| Tubingen; 45 | 29 | 16 AML, 7 ALL, 3 NHL, 2 MM, 1 CML | CD3/CD19 depletion | Flu, Thio, Mel, OKT-3 | None | Adults patients |
| Memphis; 104 | 22 | 9 AML, 9 ALL, 2 CML, 2 Ly | CD3 depletion | Flu, Thio, Mel, OKT-3 | MMF | Pediatric patients |
| Frankfurt; 46 | 59 | 15 ALL, 14 AML, 2 MDS, 13 RMS, 5 solid tumors, 10 NM | CD3/CD19 depletion | Flu, Thio, Mel | NA | Pediatric patients |
| Madrid; 47 | 29 | 18 ALL, 11 AML | CD3/CD19 depletion in 26; CD34+ selection in 3 | Flu, BU, Thio, mP | CsA alone (6) or ATG alone (8) or CsA + MTX (15) | Pediatric patients |
CML indicates chronic myelocytic leukemia; Leuk, leukemia; ML, malignant lymphoma; MM, multiple myeloma; AL, acute leukemia; Ly, malignant lymphoma; NM, nonmalignant; NR, not in remission; RMS, rhabdomyosarcoma; NHL, non-Hodgkin lymphoma; TCD, T-cell depletion; Ara-C, cytosine arabinoside; Cy, cyclophosphamide; Flu, fludarabine; BU, busulfan; Mel, melphalan; VP-16, etoposide; mP, methylprednisolone; Thio, thiotepa; CsA, cyclosporine A; MTX, methotrexate; TAC, tacrolimus; and NA, not applicable.
Immune reconstitution
Because thymic function is poor in adults, posttransplantation immune reconstitution depends for many months on peripheral expansion of mature T lymphocytes in the graft. Whether T cell–depleted full haplotype-mismatched HSCT follows myeloablative conditioning or RIC, it is associated with slow posttransplantation immune recovery because of the very small number of T cells in the BM inoculum. Thus, recipients tend to remain susceptible to life-threatening opportunistic infections, such as CMV and aspergillus. Attempts to improve posttransplantation immune reconstitution have, to date, focused on adoptive transfer of T cells.
Adoptive immunotherapy with donor T cells
Specific antipathogen immunotherapy.
Nonalloreactive, donor origin, antipathogen specific CD4+ T-cell clones were generated and successfully transferred to recipients of HLA-haploidentical HSCT.51 None of the 34 recipients of up to 1 × 106/kg anti-CMV or anti-aspergillus T cells developed GVHD. Infusion of aspergillus-specific type 1 CD4+ clones controlled aspergillus antigenemia and helped clear invasive aspergillosis in 9 of 10 patients. Immunotherapy with anti-CMV CD4+ cell clones significantly reduced CMV reactivation rates and accelerated the development of anti-CMV CD8+ clones. In other studies, refractory CMV disease was successfully cured with CMV specific donor T lymphocytes52 ; anti–human adenovirus CD4+ clones were successfully used in the haploidentical setting,53 and anti-EBV CTLs were effective as a salvage treatment for posttransplantation lymphoproliferative disorder in 6 patients who failed to respond to rituximab treatment.54
Because laboratory procedures are cumbersome and time-consuming, all these forms of immune therapy are difficult to apply as routine prophylaxis or when urgently needed on failure to respond to antiviral therapy. Genetically modified antigen-presenting cells with triple viral antigens combined with a gas-permeable culture device might markedly shorten these procedures and enable simultaneous production of CTLs against different viruses.55
To circumvent the need for individualized therapy, Haque et al used EBV-specific CTLs that were generated in advance from third-party EBV+ blood donors.56 Thirty-three organ recipients who developed EBV+ posttransplantation lymphoproliferative disorder and failed conventional treatment were treated. Complete or partial response rates were 64% at 5 weeks and 52% at 6 months with no adverse effects. Although third-party CTLs are short lived in vivo and require repeated infusions, use of “off-the-shelf” third-party CTLs could potentially treat other viral infections as well.
Using a panel of artificial antigen-presenting cells presenting different HLA determinants, Hasan et al generated, for almost every patient, a CTL line directed against the EBV or CMV peptide on at least one host HLA allele.57 Although very interesting as an approach, no clinical data are as yet available.
Another approach to circumvent cell-culture–related problems is to sort out antigen-specific T cells according to γ-IFN production. The Feuchtinger et al generated CMV-specific T cells by ex-vivo stimulation with pp65 and sorted out the antigen-specific lymphocytes according to γ-IFN production.52 Eighteen HSCT recipients with refractory CMV disease and/or viremia were treated with a mean of 21 × 103/kg CMV-specific T cells. The viral burden was cleared or significantly reduced in 83% of cases, including 2 patients with CMV encephalitis. Viral control was associated with in vivo expansion of CMV-specific T lymphocytes in 12 of 16 patients. No acute side effects or GVHD were observed, even in 11 recipients of full haplotype-mismatched transplants.
Further improvement of specific T-cell selection could be afforded using streptamers that present antigens in the desired HLA determinant. Unlike tetramers, streptamers are gradually stripped off after infusion and are therefore viewed more favorably by regulatory agencies. Proof of concept was achieved in 2 HSCT recipients with recurrent high CMV antigenemia. After a single infusion, the frequency of CMV-specific CD8+CD45RA+CCR7− effector T cells increased from 0.0% to 27.1% of all T cells. CMV antigenemia was cleared, allowing patients to discontinue antiviral drugs without toxicity or GVHD.58
Taken together, different methods for the generation of effective specific antipathogen T cells free of GVHD risk clearly offer a feasible clinical approach for mitigating posttransplantation infections.
Broad repertoire immunotherapy
An alternative to pathogen-specific therapy is adoptive T-cell immunotherapy, which provides large numbers of wide repertoire cells, mirroring the physiologic immune system. The key challenge is to infuse millions of T cells/kg in full haplotype-mismatched recipients without causing GVHD. Several strategies have been proposed:
Ex-vivo depletion of alloreactive T cells.
T-cell preparations are purged of alloreactive donor T cells by activation in mixed lymphocyte reaction against host stimulators, followed by depletion with immunotoxins, immunomagnetic selection, or FACS, all of which exploit expression of cell surface activation markers (CD25, CD69, CD134, CD137, CD147, and HLA-DR).59-63 Other methods include FasL-mediated killing of activated T cells,64 apoptosis induction by heat shock protein 90 (HSP 90),65 or anergy induction by costimulatory blockade.66
The anti-CD25 antibody conjugated to ricin toxin was first used in pediatric patients with immune deficiencies.67 More recently,68 8 children with hematologic malignancies who were given 105 T cells/kg showed significantly improved T-cell recovery, with expansion of the effector memory population lasting for at least 3 to 5 months after HSCT. The incidence of severe GVHD was low. The authors later advocated using immunomagnetic selection of CD25/CD71+ cells to remove more alloreactive cells and to increase the number of infused T cells.69
Photodynamic allodepletion provided effective and extensive depletion of alloreactive T cells while retaining broad repertoire T cells for adoptive immunotherapy.70 Safety in haploidentical transplant recipients was demonstrated in a phase 1 or 2 study with infusion of 1 to 2 × 106/kg photo-allodepleted T cells.71 An international multicenter study, designed to demonstrate efficacy, is currently under way.
Suicide gene insertion into T cells.
Bonini et al inserted the herpes simplex thymidine kinase suicide gene (TK cells) into T cells to achieve in vivo susceptibility to ganciclovir.72 In a phase 1 or 2 study,73 28 patients received approximately 10 × 106 TK cells/kg. Twenty-two patients exhibited immune reconstitution at a median of 23 days after infusion (range, 13-42 days). aGVHD (grade 1-4) in 10 patients and cGVHD in one patient were controlled by ganciclovir administration. Overall 3-year survival was 49% (95% CI, 25%-73%) for 19 patients who were in remission at transplantation. The low incidence of GVHD indicated impaired functionality of the transduced T cells, which at a dose of 10 × 106/kg would have otherwise induced a high rate of lethal GVHD. Because long-term reconstitution was largely mediated by newly emerging T lymphocytes, the authors hypothesized that TK cells triggered thymic function. A phase 3 clinical trial is now ongoing.
Combining donor Tregs and conventional T lymphocytes (Tcons).
Immunotherapy with either naturally occurring, freshly isolated donor Tregs, ex vivo expanded polyclonal, or recipient-type specific Tregs was shown in mouse models to prevent the GVHD induced by coinfused Tcons and to promote posttransplantation immune reconstitution.74-77 In a pilot study by Brunstein et al on 23 patients,78 double-unit UD-UCB transplantation was followed by infusion of 0.1 to 30.0 × 105/kg third-party unrelated cord blood Tregs, which were ex vivo expanded using anti-CD3/anti-CD28 antibody-coated Dynabeads. Patients received pharmacologic immunosuppression after transplantation. The incidence of grade 2 to 4 aGVHD was lower than in historical controls (43% vs 61%; P = .05). No significant differences emerged in relapse or infection rates. In a study from Perugia,79 28 patients with high-risk hematologic malignancies received myeloablative conditioning, followed by 2 × 106/kg freshly isolated donor Tregs. Four days later, patients received 1 × 106 Tcons and 10 × 106 highly purified CD34+ cells from full haplotype-mismatched donors. Although no posttransplantation immunosuppression was administered, the incidence of aGVHD and cGVHD was extremely low. Interestingly, the pattern of posttransplantation immune reconstitution was very different from standard T cell–depleted full haplotype-mismatched HSCT, with rapid recovery of T-cell subpopulations, development of wide T-cell repertoire, and high frequencies of pathogen-specific CD4+ and CD8+ lymphocytes. There were significantly fewer CMV reactivation episodes, with no deaths from CMV disease.
This pilot study demonstrated that naturally occurring polyclonal Tregs control the alloreactivity of up to 1 to 2 × 106/kg Tcons immunotherapy and are not associated with bystander inhibition of general immunity that would compromise response to pathogens.
However, TRM did not improve because of the clinical status of the patients at time of transplantation and regimen-related toxicity. Therefore, in an ongoing study, alemtuzumab replaced cyclophosphamide in the conditioning regimen. Preliminary results show that the incidence of life-threatening infections is greatly reduced and TRM has fallen to < 20% (M. Di Ianni, F. Falzetti, A. Carotti, A. Terenzi, L. Ruggeri, A. Pierini, V. de Fazio, V. Zoi, M. Massei, Y.R., A. Verlardi, F. Aversa, and M.F.M, Adoptive immunotherapy with Tregs and Tcons ensures low TRM and a low incidence of post transplant leukemia relapse after HLA haploidentical transplants for acute leukemia, 2011).
These pilot studies demonstrated that in vitro priming of naturally occurring CD4+CD25+FoxP3+ Tregs is not required for GVHD inhibition because activation of alloantigen-specific Tregs occurs efficiently in vivo. The number of naturally occurring Tregs that are harvested from healthy donors is sufficient to control the alloreactivity of up to 1 to 2 × 106/kg Tcons infused a few days later, and favor immune reconstitution without bystander inhibition of general immunity that would compromise response to pathogens. Thus, ex vivo expansion of donor Tregs is not a prerequisite for designing Treg-based cellular therapies
Full haplotype-mismatched HSCT as a platform for variety of clinical conditions
With its high engraftment rates, ability to prevent GVHD by T-cell depletion, and no need for posttransplantation pharmacologic immunosuppression, full haplotype-mismatched transplantation might be used in the future for the following purposes:
Haploidentical transplant as a platform for cell therapy with donor cells
Prevention of leukemia relapse by enhancing GVL effect without overt GVHD represents another major challenge that must be addressed, especially in patients transplanted in advanced-stage disease and under RIC. Specific donor antineoplastic cells would be ideal for posttransplant adoptive immunotherapy. Already at present, different modalities can be used to generate antitumor cells from host T cells, either transfected with transgenes encoding tumor-specific T-cell receptors80 or chimeric receptor-modified T cells, based on tumor-specific antibody-derived specificity (“T-bodies”).81-83 Prerequisites for any future treatment with donor-derived antileukemic T cells84 or NK cells85 are absence of GVHD and posttransplantation immunosuppression.
Recently, anti–third-party CTLs with central memory phenotype were shown to promote BM allografting.86 Furthermore, these cells exhibited a novel TCR-independent killing mechanism of human lymphoma cells, mediated by apoptosis induction after ligation of the CTL CD8 molecule to MHC class I on malignant cells. When tested in a murine model for B-cell lymphoma, these CTLs eliminated residual disease and significantly prolonged survival.87 Thus, such cell therapy, combined with mega-dose CD34+ HSCT, could offer an attractive general platform for tolerance induction as a prelude for cell therapy or organ transplantation (see below) and, more specifically, for eradicating residual disease in patients with B-cell malignancies who cannot tolerate intensive radio-chemotherapy.
Haploidentical transplants for nonmalignant diseases
Apart from transplantation in immune deficiencies,88 the use of haploidentical donors for other nonmalignant diseases is in its infancy. Sodani et al recently described a series of 22 thalassemia patients undergoing haploidentical maternal stem cell graft.89 To prevent rejection, combination of mega-dose purified CD34+ cells and highly myeloablative conditioning regimen was used. Despite this, graft rejection was reported in 6 patients and 2 patients died of opportunistic infections. The delayed immune reconstitution observed was attributed to thalassemia-related impaired thymic function and altered BM microenvironment with diminished IL-7 secretion. Clearly, the use of RIC protocols in this setting is highly desirable. Very recently, a proof of concept for the use of RIC together with high-dose posttransplantation cyclophosphamide was demonstrated in 3 patients with paroxysmal nocturnal hemoglobinuria.90
Haploidentical transplant as a platform for organ transplantation
Owen,91 Billingham et al,92 and others have established the concept that mixed hematopoietic chimerism can induce long-lasting immune tolerance toward donor cells and tissues. This has led over the years to the development of strategies to cotransplant BM and solid organ from a single HLA-haploidentical family member. Kidney transplants, followed by conditioning with total lymphoid irradiation, ATG, and transplantation of mismatched G-CSF–mobilized progenitor cells from the same kidney donor, resulted in temporary mixed chimerism and allowed for the discontinuation of immunosuppressive treatment for a limited time.93 Kawai et al demonstrated that, several months after combined BM and kidney transplants from HLA single-haplotype–mismatched donors, all immunosuppressive therapy could be discontinued without significantly affecting transplant function.94 Even though only transient chimerism was observed with the nonmyeloablative protocol used, it was sufficient to induce specific donor unresponsiveness. The mechanism of tolerance induction in their system was thought to be switching from central tolerance to peripheral mechanism that included Tregs. However, protocols that favor long-term mixed chimerism may support a more stable tolerance toward donor tissues, preventing the reversible capillary leak syndrome observed in all patients, which might indicate minor rejection episodes.
Conclusion
When high-risk acute leukemia patients do not have an HLA-matched donor, or urgently need transplantation, physicians are left with the perplexing question of what is the best alternative option, as no randomized or prospective observational studies have been conducted to date. A retrospective analysis compared the outcome of one or 2 loci mismatched UD-UCB or 8/8 HLA allele MUD transplantation in children (age < 16 years). Survival rates overlapped, whereas relapse rates were not significantly different.95 Consequently, in the absence of 8/8 HLA allele matched MUD, 4 to 6/6 HLA Ag-matched UBCT offers an attractive option for pediatric patients. The use of UCB transplants in adults is limited by the cell dose. Such limitation has been overcome by infusing 2 UCB units, which improved engraftment and DFS rates.2
Full haplotype-mismatched T cell–depleted mega-dose transplantation offers several advantages. In children, encouraging results have been achieved in European centers with experience in the field.32,46,96 However, a larger experience has been obtained in adults. Transplants in several European centers resulted in 43% DFS in AML and 30% in ALL patients who were transplanted in any CR.29 In the Perugia series, the probability of EFS in AML increased to more than 65% with transplantation from an NK-alloreactive donor, who can be found for almost 50% of patients. Because these results are in the range of best survival rates after well-matched MUD transplantation for patients with high-risk AML in first CR, they should encourage greater use of haploidentical transplants for patients who do not have a matched donor. Indeed, with a follow-up of up to 17 years, long-term survivors of haploidentical T cell–depleted transplants enjoy a normal life with no cGVHD.29
Furthermore, comparing full haplotype-mismatched and MUD transplantation, one should note that virtually all patients receive the haploidentical graft with no undue delay, although for various reasons (inevitable time lapse of donor search and bone marrow harvest) many patients relapse and die while awaiting a MUD transplant.
Because approximately half the patients who start a donor registry search actually undergo transplantation, omission of intention to treat from the calculation of final outcomes in most studies creates a marked bias in favor of MUD transplants. Therefore, intention-to-treat trials are needed to establish the most appropriate algorithm for alternative donor selection.
Attention at present is focused on the use of haploidentical T cell–depleted HSCT without any posttransplantation immunosuppression as a platform for adoptive T-cell immunotherapy for hastening posttransplantation immune reconstitution, reducing TRM and enhancing the GVL effect. The availability of the haploidentical donor is therefore of even greater importance when considering the possibility of using the same donor for any developed cell therapy.
In parallel, attempts are ongoing to extend the use of haploidentical transplant for elderly and for patients with significant comorbidities, using RIC together with either ex-vivo T cell–depleted grafts, or T cell–replete grafts and posttransplantation cyclophosphamide to prevent GVHD.
In conclusion, this review has illustrated how full haplotype-mismatched transplantation has evolved from a last-attempt option for end-stage patients, to an established form of treatment that must be considered for selected patients with acute leukemia in first remission and high risk of relapse.
Note added in proof:
Y.R. acquired a consulting position with Cell Source on November 1, 2011. He receives funding for research and shares.
Acknowledgments
The authors thank Prof Jacob M. Rowe, Prof Franco Aversa, Prof Andrea Velardi, Dr Avichai Shimoni, and Dr Geraldine A. Boyd for helpful suggestions and critical reading of the manuscript.
Authorship
Contribution: Y.R., D.H., and M.F.M. reviewed the literature and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yair Reisner, Department of Immunology, Weizmann Institute of Science, Herzl Street 1, Rehovot 76100, Israel; e-mail: yair.reisner@weizmann.ac.il.