Question: When is defective memory cell function a good thing? Answer: When effector memory T (TEM) cells are administered in allogeneic hematopoietic cell transplants (HCTs). TEM cells are defective in their capacity to induce GVHD. In this issue of Blood, Juchem et al employ a clever preclinical allogeneic HCT strategy to gain insight into why this is so.1 After transplantation of univariate naive(TN), central memory (TCM), and TEM CD4 T cells, the investigators conclude that repertoire differences cannot explain the TEM defect. Interestingly, functional deficits intrinsic to TEM cells appear to contribute to their impotence.2
The transplant field continues to search for strategies to prevent GVHD after allogeneic HCT while maintaining the beneficial affects of donor T cells including engraftment, antitumor responses, and immune reconstitution. A number of groups have demonstrated that CD4 and CD8 TEM cells differ from other subsets because unlike TN or TCM cells, the former fail to induce significant GVHD in several experimental model systems.1-6 Despite such observations, evidence continues to accumulate that TEM cells provide antitumor reactivity, the raison d'etre of most allogeneic HCTs.7,8 Thus, understanding why TEM cells behave differently from other subsets could lead to promoting the use of multiple T-cell populations to minimize complications and maximize the benefits of allogeneic HCT.
Several explanations have been proposed to account for the poor GVHD activity by TEM cells. For example, their migration and priming could be deficient because these cells lack CD62L and CCR7 to gain entry into secondary lymphoid tissue. Thus, relative exclusion from sites where efficient allo-activation occurs, that is, lymph nodes, might dampen their ability to induce GVHD. However, GVHD occurs in the absence of lymph nodes as well as with T cells lacking CD62L and CCR7 indicating that trafficking alone cannot account for the failure of TEM cells to cause GVHD.9,10 Another difference between TEM cells and naive T-cell populations that could influence their ability to induce GVHD may be their TCR repertoire, as memory cells have been proposed to contain significantly fewer overall alloreactive specificities.11 Finally, TEM cells may also be limited in their GVHD response as a consequence of diminished clonal expansion and/or weak pathogenic effector functions.
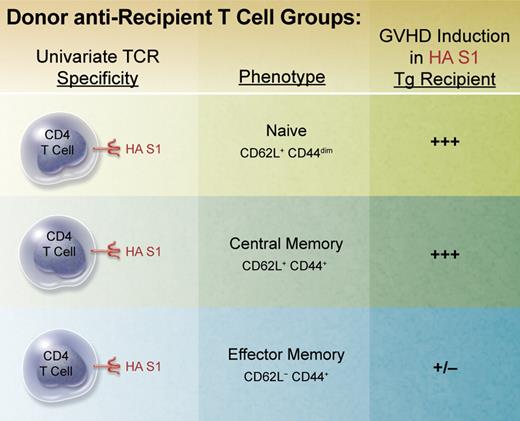
In an attempt to shed light on the contributions of repertoire and function, Juchem et al developed an elegant approach to eliminate repertoire differences between populations of donor T cells administered for transplantation. Using TCR transgenic CD4 T cells specific for a viral hemagglutinin (HA) antigen, the authors directly obtained naive T cells and generated memory populations using ex vivo stimulation followed by transfer, that is, parking, in syngeneic RAG-deficient recipients before cell sorting for TEM and TCM populations (see figure). Conditioned recipients expressing the defined viral HA epitope in all tissues were transplanted to examine T-cell activation, expansion, function, and GVHD induction. In contrast to both TN and TCM cells, which readily induced weight loss, lethality, and pathology characteristic of GVHD, donor TEM cells were clearly defective in this capacity on a per cell basis. Hence, given the identical antigen specificity of all 3 populations, repertoire in this model could be eliminated as mea culpa for the observations.
BALB/c TS1 transgenic RAG-deficient mice possess a univariate T-cell receptor (TCR) specific for the immunodominant epitope of influenza A virus PR8 hemagglutinin (HA) S1 determinant (aa 110-119) presented in the context of MHC class II IEd. Naive donor CD4 T cells were isolated via flow sorting from unmanipulated TS1 mice. Memory populations were generated by isolating and stimulating these T cells in vitro for several days with HA S1 peptide and adoptively transferring them into BALB/c RAG-deficient mice for several months. Memory cell populations were then obtained by flow sorting. These subsets were then individually transplanted together with bone marrow from BALB/c RAG-deficient donors into lethally conditioned BALB/c transgenic mice (BALB/c HA104) expressing the HA S1 determinant throughout.13 Recipient mice were monitored, scored, and evaluated for clinical and histopathologic changes characteristic of GVHD. Professional illustration by Alice Y. Chen.
BALB/c TS1 transgenic RAG-deficient mice possess a univariate T-cell receptor (TCR) specific for the immunodominant epitope of influenza A virus PR8 hemagglutinin (HA) S1 determinant (aa 110-119) presented in the context of MHC class II IEd. Naive donor CD4 T cells were isolated via flow sorting from unmanipulated TS1 mice. Memory populations were generated by isolating and stimulating these T cells in vitro for several days with HA S1 peptide and adoptively transferring them into BALB/c RAG-deficient mice for several months. Memory cell populations were then obtained by flow sorting. These subsets were then individually transplanted together with bone marrow from BALB/c RAG-deficient donors into lethally conditioned BALB/c transgenic mice (BALB/c HA104) expressing the HA S1 determinant throughout.13 Recipient mice were monitored, scored, and evaluated for clinical and histopathologic changes characteristic of GVHD. Professional illustration by Alice Y. Chen.
An important finding by Juchem et al was that during the first month after HCT, in most target tissues including spleen, liver, and bone marrow, similar numbers of TEM cells compared with TN and TCM cells could be recovered. Thus, overall poorer survival, reduced expansion, and engraftment did not appear to contribute to TEM cells' deficiency. However, examination of one tissue, the colon, did yield intriguing results: unambiguously decreased numbers of CD4 TEM cells versus the other populations. The reduction reached ∼ 20 times two weeks after transplant. Consistent with such observations, pulse label experiments demonstrated the frequency of colonic TEM cells in division was also far less than TN cells. This latter finding highlights that in addition to secondary lymphoid organs, further stimulation and division clearly occurs in target tissues weeks after transplant. Based on the known significance of the GI tract in the development of GVHD, these observations are not likely coincidental. Diminished inflammation in the GI tract, particularly early after transplant, is clearly recognized as an important venue to contain development and propagation of GVHD. In total, these findings support the hypothesis that the transgenic CD4 TEM cells induce early GVHD activity but fail to sustain responses leading to full-fledged and severe disease. Of note, cotransfer experiments demonstrated that the presence of TN cells together with TEM cells, a scenario that would typically reflect the heterogenous populations of T cells applied in the clinic, resulted in further suppression of TEM expansion in the colon as well as in the spleen. Why or how alloreactive TN cells may regulate TEM cells and whether Tregs are involved will await further investigation.
What is known regarding function by TEM cells in this model? Cytokine analysis demonstrates that the progeny of these cells in the colon as well as spleen exhibit diminished production of IFN-γ evidenced by both overall frequency and the magnitude of IFN-γ produced. Although small decreases in IL-2 and IL-17 were also noted by cells in the colon, the overall low frequency of producing cells suggests a less important role here versus IFN-γ. However, reduced IL-2 production was also apparent by TEM progeny in the spleen, where a significant frequency of IL-2–producing cells was detected. Why the progeny of TEM cells exhibit diminished functional responsiveness (and expansion in the colon) is presently unknown. One clue may reside in the elevated PD-1 expression observed on TEM progeny a month after transplant whose signaling may contribute to exhaustion and reduced functional capacity. Thus in the colon, diminished TEM cell numbers together with their diminished cytokine production predicts the decreased pathology observed in this tissue.
Models of polyclonal T cell–induced GVHD have also contributed to our understanding of the roles of repertoire and intrinsic defects with respect to TEM cells' inability to sustain GVHD. Interestingly, prior studies employing polyclonal donor T cells in GVHD models reported recovering fewer TEM cells versus TN cells. This contrasts with the virtually equivalent numbers detected in all tissues examined except within the colon in the transgenic model.8,9 Juchem et al propose that the polyclonal findings may reflect the greater levels of alloreactive T cells in the TN populations, perhaps together with inhibition of TEM cells when they are cotransplanted with TN cells. Thus, while repertoire could play a role should it differ between TN cells and TEM cells, the findings by Juchem et al illustrate that regardless of repertoire, there are fundamental differences between TEM and TN cells. To date, accumulating evidence supports the notion that in preclinical mouse models, TEM cells may be pretty, pretty good for allogeneic BMT administered as therapy to treat hematopoietic malignancies. Nonetheless, we must still curb our enthusiasm regarding translation to the clinic as transplanters await further definition as well as purification of the complex human T-cell memory pool.12
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal