Abstract
Abstract 953
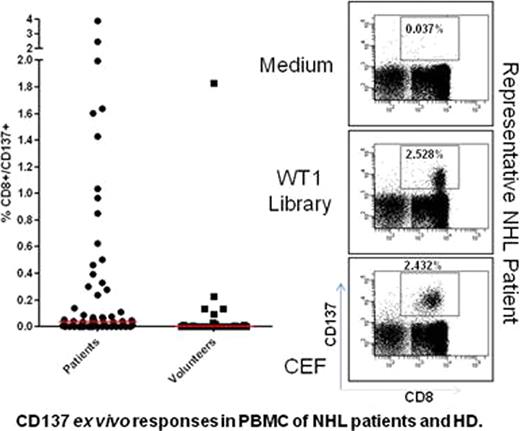
is expressed in many solid tumors and hematologic malignancies and serves a physiological role in the maintenance of the tumorigenic phenotype. Immunologic recognition of WT1 has been observed in patients with myeloid and lymphoid leukemia, solid tumors, and even in healthy adults. However, little is known about WT1 expression or WT1-specific immune responses in patients with non-Hodgkin lymphoma (NHL). Current therapeutic modalities for NHL patients are predominantly based on the highly successful application of the anti-CD20 mAb (Rituximab™) or radioactive derivatives (Zevalin™ or Bexxar™) combined with standard chemotherapy. Recent approaches have focused on generating NHL-specific idiotypes as an example of personalized medicine. However, harnessing a T cell response to malignant B or T cells in NHL patients has not succeeded, despite its attractiveness as a universal therapeutic option. We developed a detection system for WT1-specific T cells that is HLA independent by constructing a peptide library of 15mers (11 residues overlapping) corresponding to full length WT1 antigen. We analyzed peripheral blood mononuclear cells (PBMC) after priming with the peptide library for immune responses to the WT1 antigen detectable by flow cytometry based on the CD137 activation marker. Sixty-three NHL patients and 30 healthy donors (HD) were recruited under IRB-approved protocols. The cohort included patients who received allogeneic stem cell transplantation (alloHCT: n=42) or chemotherapy/autologous HCT (n=21). We observed that WT1-specific ex vivo CD8+ T cell responses were significantly greater in our NHL patient cohort (median 0.035%) compared to our randomly chosen HD cohort (0%, p=0.0013)(Figure 1). These WT1-specific CD8+CD137+ T cells co-expressed CD45RA and CD57 surface markers, consistent with them being a TEMRA (Terminal Effector Memory, RA+) population of highly differentiated T cells. Twenty-four of 43 patients (55.8%) with high/intermediate-grade NHL had a positive CD8+ T cell response (CD8+CD137+ ≥0.05%) to the WT1 antigen compared to four of 20 patients (20%) with low-grade NHL (p=0.011). None of the other clinical variables such as patient age, treatment modality (alloHCT vs. autoHCT or chemotherapy), disease status (CR vs. non-CR), prior acute GVHD (only in alloHCT, grade 0-I vs. II–IV) were significantly associated (p≤0.05) with WT1-specific CD8+ T-cell response in NHL patients when we conducted a multivariate analysis. PBMC from one HD and 12 NHL patients were expanded using in vitro stimulation (IVS) with the WT1 peptide library. All PBMC samples expanded in culture after 10–14 days by single IVS (median 7-fold expansion). We observed significantly higher IFN-γ responses in CD8+ T cells when IVS cells were re-stimulated with the WT1 library (median=3.0%, range: 0.08–5.5%) compared with the medium control (median=0.39%, range: 0.05–1.7%, p=0.002). Remarkably, using standard in vitro techniques, the expanded PBMC were cytotoxic to WT1-peptide library-loaded T2 cells and autologous EBV-transformed B-cell lines derived from patients enrolled in the study. These results challenge the notion that central tolerance mechanisms eliminate high affinity WT1-specific T cells that kill tumor cells.
In order to distinguish whether WT1-specific T cells are maintained in NHL patients because of an aberration of tolerance mechanisms or the presence of WT1 antigen as a stimulator, we used sensitive real time quantitative PCR (RT-qPCR) methods to detect transcripts in LN from NHL patients and HD. We succeeded in detecting WT1 mRNA transcripts in diseased lymph node (LN) tissues of NHL patients utilizing RT-qPCR technology. There was significantly greater WT1 mRNA in NHL LN (n=6) with an average copy number ratio of 39 (WT1/c-ABL × 104) compared with 0.66 in non-disease LN obtained from individuals without a cancer diagnosis (n=7, p<0.05). This exciting result strongly implicates WT1 antigen expression as a driving force of WT1-specific T cell expansion in NHL patients. In summary, ex-vivo WT1-specific CD8+ T cell responses can be detected in NHL patients, expand in vitro, produce IFN-γ, and are cytotoxic. These results are the first demonstration of significant T cell reactivity against the WT1 antigen in NHL patients. The potential for developing the WT1 antigen as a target for immunotherapy in NHL patients deserves further exploration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal