Abstract
Abstract 950
Diffuse large B-cell lymphoma (DLBCL) can be categorized by its cell of origin (CoO) as either being derived from a germinal center B-cell (GCB) or activated B-cell (ABC). Primary mediastinal DLBCL represents a third, distinct entity. This classification was initially defined by gene expression profiling (GEP), which remains the gold standard for such determination. Determination of CoO will likely become the basis for patient selection for clinical trials of targeted therapies. Several algorithms and methods have been developed that use immunohistochemistry (IHC) to differentiate GCB-DLBCL from non-GCB DLBCL. These include the Hans algorithm (utilizes staining for CD10, Bcl-6 and Mum1), the Choi algorithm (utilizes additional staining for GCET1 and FoxP1) as well as the Tally method (does not use Bcl-6 and utilizes LMO2 as a tie-breaker stain for otherwise equivocal cases). Recently, it has been recognized that IHC approaches to assign CoO may not be reproducible even at highly experienced laboratories. We sought to determine the performance of these IHC assays in our laboratory as a necessary step in developing trials based on CoO stratification.
We reviewed 108 adult (age ≥18) cases of de novo DLBCL, the majority of which were treated with chemoimmunotherapy (R-CHOP or R-CVP) at the Cleveland Clinic from 2000–2010. Diagnostic biopsies were available for all cases. IHC staining was performed on tissue microarrays (TMAs), and published algorithms (Hans, Choi and Tally) were applied to categorize cases as GCB or non-GCB. In addition, gene expression profiling was completed in a subset of these cases, for which frozen tissue was available. A linear predictor score for gene expression profiling (GEP) was used to assign cases in 31 of 33 cases with 2 technical failures at the array stage (overall success rate 84.8%). Clinical details including age, sex, International Prognostic Index (IPI) stage at diagnosis, treatment, progression free survival (PFS) and overall survival (OS) were captured for 69 of the 108 patients. Actuarial survival analysis was performed according to the Kaplan and Meier method, and the curves compared by the log-rank test.
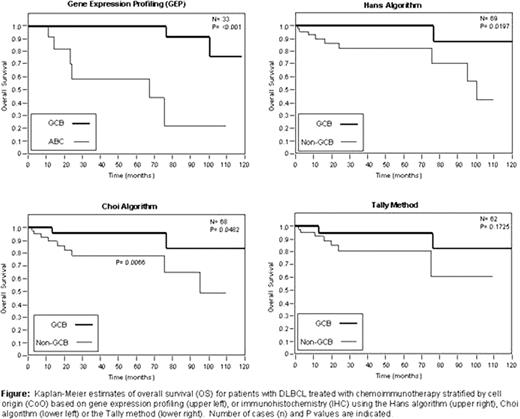
For the 69 patients with adequate clinical follow-up, the median age was 64 years old (range 18–88). There were 49% males and 51% females. The distribution of patients with stage I, II, III, and IV disease at the time of diagnosis was 20%, 14%, 20%, and 32% (14% had unknown stage). The 5-year overall survival of patients was 88%. Results of the Hans algorithm, Choi algorithm and Tally method were interpretable in 98 (90.7%), 95 (87.9%) and 88 (81.5%) of 108 cases, respectively. Inability to assign subtypes was due to suboptimal staining of the TMA (tissue loss or poor staining of an individual core). Using GEP to assign CoO, 42% of cases were classified as GCB, 42% as ABC and 14% were unclassifiable. The sensitivities of the Hans, Choi and Tally approaches to identify the CoO predicted by GEP were 0.83, 0.83, and 0.58 for correctly identifying GCB cases, respectively, and were 0.70, 0.70 and 0.80 for identifying non-GCB cases, respectively. The positive predictive values of the Hans, Choi and Tally approaches were 0.83, 0.83, and 1.0 for GCB and 0.78, 0.78, and 0.89 for non-GCB. As shown in the figure, 5-year overall survival was significantly superior for GCB relative to ABC cases using GEP (100% vs. 58.9%, P < 0.001) and for GCB vs. non-GCB cases for the algorithms of Hans (100% vs. 82.3%, P = 0.0197) and Choi (95.6% vs. 78.0%, P = 0.0482). The Tally method was not predictive of outcome, possibly due to insufficient power (5-year OS 94.4% for GCB vs. 80.7% for non-GCB, P = 0.1725). Similar findings were observed for progression-free survival.
CONCLUSIONS: The Hans and Choi algorithms are reasonable methods for identifying PFS and OS differences based on CoO for de novo DLBCL treated with chemoimmunotherapy. The positive predictive value is universally high for all algorithms tested, but the sensitivity of IHC for identifying CoO was fair, particularly for the Tally method. IHC represents a valid biomarker to identify non-GCB cases. Clinical trials of DLBCL that stratify patients by IHC are feasible provided the performance characteristics of the algorithms are taken into consideration during study design.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal