Abstract
Abstract 941
Algorithms for relapse risk prediction, kinetics of hematological relapse (HR) following a molecular relapse (MR) and intervention based on these results have been well studied for patients with acute promyelocytic leukemia (APL) treated with conventional all-trans retinoic acid (ATRA) plus chemotherapy based regimens. It is recognized that the kinetics of leukemia clearance with the use of arsenic trioxide (ATO) in induction is significantly different from that of ATRA alone or ATRA plus chemotherapy combinations. Extrapolation of data generated from ATRA plus chemotherapy regimens may potentially not be valid when applied to regimens that use ATO in up front therapy.
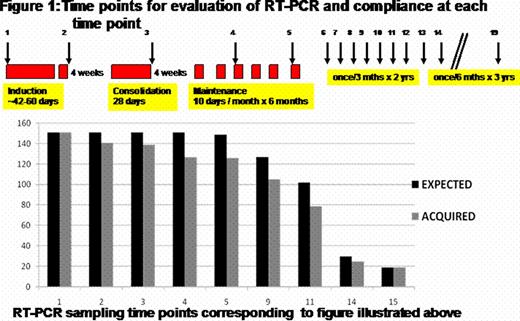
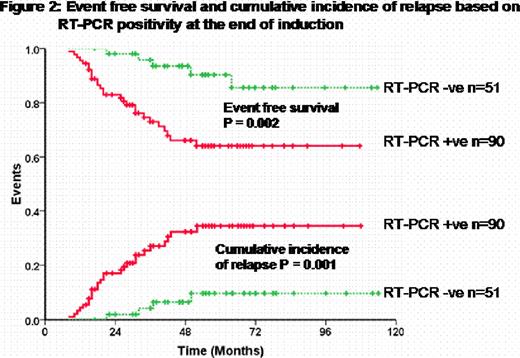
At our center we undertook a prospective minimal residual disease (MRD) detection study in APL treated with ATO. 151 patients who achieved hematological remission (CR) were followed up serially for MRD detection by peripheral blood RT-PCR. All patients achieved a molecular remission (CRm) prior to starting maintenance therapy. Figure 1 summarizes the time points for analysis, compliance and RT-PCR positivity. An RT-PCR was positive in 90 (64%) at the end of induction which was associated with a significant increase in the risk of relapse. On a multivariate analysis after adjusting for conventional risk factors that predict relapse, an RT-PCR positivity at this time point was the only parameter that retained statistical significance (RR=3.82; 95%CI=1.2–12.8; P-value=0.03). The event free survival (EFS) and cumulative incidence of relapse (CIR) for those who were RT-PCR positive was significantly worse than those who were negative as illustrated in figure 2. Further analysis revealed that none of the low risk patients (WBC count at diagnosis <5 × 109/Lt and platelet count >20 × 109/Lt) who were RT-PCR negative at the end of induction relapsed while 4 (10%) of the high risk group patients who were RT-PCR negative, at this time point, relapsed. There were a total of 31 (20.5%) hematological relapses, only 3 of these relapses were beyond 3 years from completion of maintenance. An RT-PCR was positive in the 3–4 months period prior to a HR in 15/31 (48.3%), negative in 10/31 (32.2%) and was not done in this time frame in 6/31 (19.3%). An additional 7/151 (4.6%) were transiently RT-PCR positive but did not have a HR at a median follow up of 50 months (range: 21–87) from a positive test. The overall sensitivity, specificity and positive predictive value of using an RT-PCR for successful MRD detection prior to a frank HR was 62.5%, 93.3% and 65.2% respectively. Retrospective RQ-PCR analysis at the same time points revealed that with an RQ-PCR the sensitivity would be further improved to 83.3%.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal