Abstract
Abstract 934
Induction chemotherapy in acute myeloid leukemia (AML) has been shown to successfully induce complete remission in over 70% of patients. However, a majority of patients experience subsequent relapse. Assessment of minimal residual disease (MRD) by flow cytometry at time of aplasia, after induction and after consolidation therapy has been shown to be of prognostic relevance for relapse free survival (RFS) and overall survival (OS). However, studies utilizing MRD diagnostics to guide therapeutic decisions in adult AML (excluding APL) are yet to be performed.
From the database at the Laboratory of Leukemia Diagnostics at our clinic datasets of 583 patients with newly diagnosed AML treated between 2000 and 2011 were analyzed. Patients with biphenotypic acute leukemia, M3 according to FAB classification, as well as those not treated with intensive induction chemotherapy were excluded. To be eligible for further analysis, at least two samples of bone marrow blood (at primary diagnosis and at one further timepoint during or after treatment) had to be available for MRD assessment by 3-color-flow cytometry at our laboratory. Cytogenetic and molecular risk stratification was performed at our clinic and assigned in accordance to the European LeukemiaNet (ELN) guidelines. We used Cox Proportional Hazards Regression to determine prognostic factors for OS and RFS and Kaplan-Meier estimator to determine OS and RFS of the proposed score.
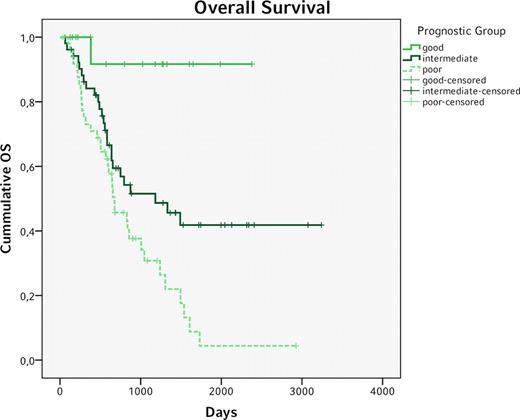
Data of 217 Patients fulfilled the inclusion criteria and were therefore eligible for further analysis. 171 (78,8%) patients achieved CR after induction therapy. Of these patients, 120 had flow cytometry data available at time of aplasia and were included in further analysis. The median age was 54,5 y and the median OS 1007 days. Here, only “favorable” ELN risk stratification was associated with significantly longer OS (favorable vs. intermediate-I, Intermediate-II & adverse, Hazard Ratio, HR 0,36, 95% CI 0,19–0,69, p=0,0019), whereas RFS did not yield a significant difference (HR 0,64, 0,37-1,13, p=0,125). Age > 60y was associated with significantly shorter OS (HR 2,07, 1,23-3,47, p=0,0058) and RFS (HR 1,83, 1,11-3,01, p=0,018). And though leukemia-associated phenotypes (LAIP) ≥0,15% at time of aplasia were not predictive of OS (HR 1,32, 0,79–2,23, p=0,293) they were highly predictive of shorter RFS (HR 2,15, 1,30–3,55, p=0,003). Combining these three factors in a simple prognostic score (ELN risk group “favorable” = 0 points, “intermediate-I”, “intermediate-II” or “adverse” = 1 point; age > 60y = 1 point; LAIP at time of aplasia ≥0,15% = 1 point, see table I) identified three distinct groups (0 points: good, 1 point: intermediate, 2–3 points: poor, see table II) which were predictive of both OS and RFS (see figures 1 and 2). Interestingly, this score was capable of identifying a small group of patients with a very good prognosis (n=18, median OS and RFS not reached after >6 years) while at the same time equally dividing up the remaining patients within the intermediate and poor prognosis group (n=52 vs. 50, median OS 1182 vs. 677 days, median RFS 1180 vs. 334 days).
Overall Survival for the good, intermediate and poor prognosis group (good vs. intermediate vs. poor p<0,015)
Overall Survival for the good, intermediate and poor prognosis group (good vs. intermediate vs. poor p<0,015)
Relapse free survival for the good, intermediate and poor prognosis group
MRD based therapeutic decisions and risk-adapted therapy have long been suggested in management of adult AML. Here, we propose a prognostic score for patients with AML achieving CR under intensive induction chemotherapy. The addition of MRD Flow to established genetic prognostic markers as well as age improves the prediction of relapse free and overall survival. Application of this score in therapeutic decisions could assist the treating physician and avoid over-treatment. To further evaluate our proposed prognostic score, it has to be applied in a prospective study for further evaluation and determination of its clinical significance. These data will be the basis for therapeutic trials guided by MRD assessment.
Prognostic Score Calculation
| . | 0 . | 1 . |
|---|---|---|
| ELN | favorable | intermediate-I, intermediate-II, adverse |
| Age | ≤60 | >60 |
| LAIP at aplasia | <0,15% | ≥0,15% |
| . | 0 . | 1 . |
|---|---|---|
| ELN | favorable | intermediate-I, intermediate-II, adverse |
| Age | ≤60 | >60 |
| LAIP at aplasia | <0,15% | ≥0,15% |
Prognostic Groups
| Score . | Prognostic group . |
|---|---|
| 0 | Good |
| 1 | Intermediate |
| 2–3 | Poor |
| Score . | Prognostic group . |
|---|---|
| 0 | Good |
| 1 | Intermediate |
| 2–3 | Poor |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal