Abstract
Abstract 932
Large Granular Lymphocyte Leukemia (LGLL) is a chronic lymphoproliferative syndrome of clonal mature T or NK cells frequently associated with peripheral cytopenias including neutropenia and anemia. The ability of LGLL cells to lyse pro-erythrocytes, the association with secondary autoimmune disorders, and the chronically activated state of LGLL clones suggest that the disease is secondary to a systemic reactive process. Yet, the mechanism causing severe and chronic cytopenias remains unresolved. Because LGLL cells heavily reside in the bone marrow and the bone marrow microenvironment plays such a large role in supporting hematopoiesis, we hypothesized that constituents of the bone marrow microenvironment may be dysfunctional in LGLL patients, and contribute toward the pathogenesis of LGLL and the devolvement of cytopenias.
To address this hypothesis, bone marrow core biopsies, aspirates, and peripheral smears taken from 24 patients diagnosed with LGLL were analyzed retrospectively by three independent pathology reviews. Reticulin and trichrome stains revealed clinically relevant myelofibrosis in 21 patients at the time of diagnosis. Of these, 15 had severe myelofibrosis (MF2-3, semi-quantitative grading). The severity of myelofibrosis correlated with neutropenia, anemia, splenomegaly, secondary autoimmune disorders, and the degree of LGLL bone marrow infiltration. The severity of fibrosis also correlated with novel disease aspects reported here for the first time, including increased pseudo-Pelger-Huet and immature neutrophils in peripheral smears, and increased monocytes in the bone marrow and periphery.
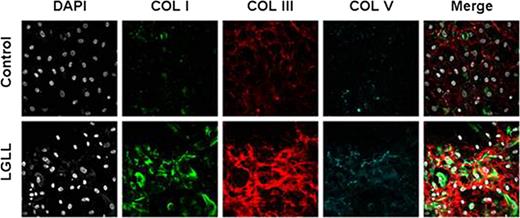
Because myelofibrosis is so strongly associated with disease severity, we sought to understand the pathogenesis of medullary fibrosis using primary mesenchymal stem cell (MSC) cultures isolated from bone marrow aspirates of LGLL patients or healthy controls. Patient MSC cultures displayed abnormal morphologies, impaired growth kinetics, and reduced growth potential compared to controls, suggesting that patient MSCs may be prematurely senescent. Microarray analysis showed global gene expression changes as healthy MSCs expand in culture including increased expression of pro-hematopoietic cytokines and autocrine growth factors vital to MSC self-renewal such as basic fibroblast growth factor (FGFb) and leukemia inhibitory factor (LIF). Patient MSC cultures appeared incapable of these gene expression changes, instead maintaining low levels of pro-hematopoietic cytokines, FGFb, and LIF expression, while maintaining heightened collagen expression. Cytokine secretion was confirmed using ELISA, and immunofluorescent staining of collagen on MSC cultures verified abnormally elevated collagen deposition from patient MSCs. In particular, collagen I and collagen III matrices were heavily deposited over patient MSCs reiterating the increased trichrome and reticulin staining seen in patient bone marrow biopsies. In an effort to rescue senescence, exogenous FGFb and LIF were added to patient MSCs. FGFb, but not LIF, completely restored the growth kinetics, growth potential, and morphology of these cells. Moreover, collagen deposition of patient MSCs was comparable to healthy donors after exposure to FGFb.
MSCs from Patient Bone Marrow Deposite increase collagen matrix compared to healthy controls.
MSCs from Patient Bone Marrow Deposite increase collagen matrix compared to healthy controls.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal