Abstract
Abstract 881
The Japan Clinical Oncology Group (JCOG)-Lymphoma Study Group (LSG) conducted 3 clinical trials in the 1990s for aggressive ATL, i.e. acute, lymphoma, and unfavorable chronic types, associated with human T-lymphotropic virus type-I. The prognosis of aggressive ATL patients (pts) is still poor, but the 5-year survival rate was improved from 5% in the 1980s to 15% in a JCOG study in late 1990s. To characterize long-term survivors and to develop a new predictive model for aggressive ATL, we performed a combined analysis of enrolled pts in the 3 JCOG studies.
A total 276 pts with aggressive ATL were registered in 3 consecutive JCOG-LSG trials exclusively against aggressive ATL, i.e. JCOG9109, a phase II (pII) study of LSG11 consisting of pentostatin, VCR, ETP, PSL and DOX; JCOG9303, a pII study of LSG15 consisting of VCAP-AMP-VECP; and JCOG9801, a pIII study comparing modified (m) LSG15 with CHOP-14. The pts were analyzed for their prognosis, the effects of central nervous system (CNS) prophylaxis in the protocol treatment and allogeneic hematopoietic cell transplantation (allo-HCT) after the protocol treatment, and the outcome of long-term survivors with a follow-up survey. To build a predictive index, multivariate analyses with a Cox proportional hazard model were conducted using the test sample of 193 pts without missing covariate data from the entire cohort of 276 pts. Validation was carried out using external data for 136 pts, who were independent from those included in the JCOG studies.
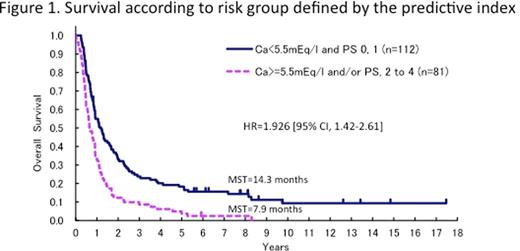
The MST, 5- and 8-yesr OS rates of the entire cohort of 276 pts were 10.9 months, 13.6% and 10.0%, respectively. The median and longest follow-up periods were 10.9 months and 17.5 years, respectively. The differences of CNS prophylaxis by each regimen did not significantly affect the CNS relapse rate. We analyzed 269 eligible pts (acute 170, lymphoma 78, chronic 21). In 67 pts who survived for more than 2 years, there were 38 (22%) pts with acute type, 21 (28%) with lymphoma type and 5 (24%) with chronic type. The major proportion of relapse in pts with acute type occurred within 2 years after initiating chemotherapy, and occurred earlier than in pts with other types. In 37 pts who survived for more than 5 years (acute 22 (13%), lymphoma 8 (10%), chronic 7 (33%)), there were no disease-related deaths among lymphoma type pts, which was in contrast to the 10 disease-related deaths that occurred in pts with acute or chronic type during the follow-up period. The numbers of pts achieving CR and the other response category at the end of the protocol treatment were 24 and 13 in the 37 pts who survived for more than 5 years, respectively, and 11 and 1 in the 12 who survived for more than 10 years, respectively. Six of the 37 pts who survived for more than 5 years and 2 of 12 who survived for more than 10 years had received allo-HCT. In a test sample of 193 pts, two unfavorable factors associated with OS, high calcium (Ca) level and ECOG performance status (PS) of 2 to 4, were identified. The hazard ratio (HR) [95% confidence interval (CI)] of Ca and PS were 1.574 [1.09–2.28] and 1.554 [1.12–2.16], respectively. Considering the potential for clinical usage, four categories using these two factors were combined into a predictive index with 2 strata, thus resulting in pts with both Ca<5.5 mEq/l and PS 0, 1, and 7.9 months, 3.7% and 2.5% in those with Ca>=5.5 mEq/l and/or PS 2 to 4, respectively (Fig 1). The HR [95% CI] was 1.926 [1.42–2.61]. The predictive model was reproducible in the validation set.
There were no disease-related deaths in 8 lymphoma type pts who survived for more than 5 years, suggesting that about 10% of pts with lymphoma type may be curable. We developed a predictive model for aggressive ATL based on Ca and PS, however, the MST was only 14.3 months even in the better prognostic group. To further improve the outcome, a pII trial of VCAP-AMP-VECP followed by allo-HCT (JCOG0907) and a randomized pII study of VCAP-AMP-VECP with or without anti-CCR4 antibody are underway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal