Abstract
Abstract 855
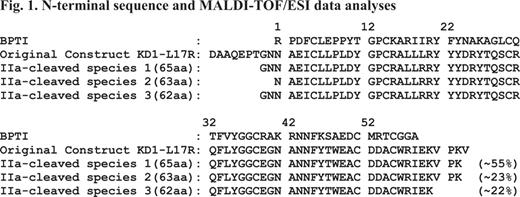
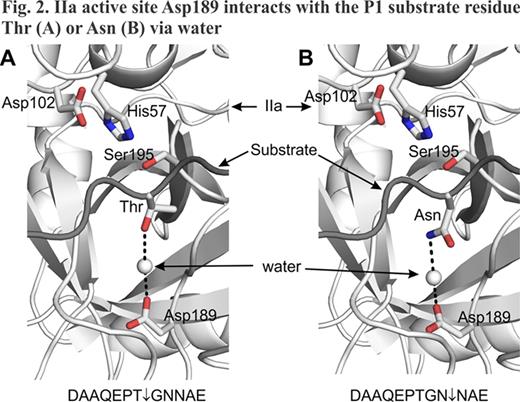
Previously, we demonstrated that changing residue Leu17 (BPTI/Aprotinin numbering) to Arg in Kunitz domain 1 (73-residue KD1-L17R) of TFPI-2 abolishes its anticoagulant functions and enhances its plasmin inhibition (Bajaj et al., J Biol Chem 286, 4329–4340, 2011). In that study we used the entire KD1 domain, which in addition to the core structural homologous region of BPTI (58 residues) included 9 residues on the N-terminal and 6 residues on the C-terminal side of the protein. Conformation of these 15 residues may be different in the isolated KD1-domain as compared to the complete TFPI-2 molecule. Thus, these residues could be potentially immunogenic. To address these concerns, we investigated weather N- and C-terminal regions of 73-residue KD1-L17R could be cleaved upon prolonged incubation with thrombin (IIa). Incubation of 73-residue KD1-L17R with IIa for 72 hrs yielded smaller version(s) of KD1-L17R as analyzed by SDS-PAGE. N-terminal sequence and MALDI-TOF/ESI mass spectrometry analyses revealed three closely related species present in the truncated KD1-L17R preparations (Fig. 1). Species 1 has Gly-Asn-Asn as the amino terminus and Val-Pro-Lys as the C-terminus. Species 2 and 3 are similar to species 1 except species 2 is produced after losing Gly and Asn from the N-terminus, whereas species 3 is produced after losing Val-Pro-Lys from the C-terminus. Thus, all three species have the intact core Kunitz domain with minor variations at the N- and C-terminus regions. Further, these species are cleaved at the viable albeit very slow IIa-cleavage sites; herein, these species are collectively referred to as truncated KD1-L17R. A plausible mechanism for proteolysis at these cleavage sites is shown in Fig. 2.
Similar to the 73-residue KD1-L17R, the truncated preparations did not inhibit (Ki > 3 μM) plasma kallikrein, factor (F) XIa, FVIIa/soluble tissue factor, FXa, activated protein C, tissue plasminogen activator (tPA), IIa and IIa/soluble thrombomodulin. Importantly, the truncated KD1-L17R preparations inhibited plasmin with Ki ∼1.2 nM. Further, the truncated KD1-L17R inhibited tPA-induced plasma clot fibrinolysis with an apparent IC50 of ∼0.37 μM, a value similar to that obtained with the 73-residue KD1-L17R and BPTI. Two lysine analogues, Epsilon amino caproic acid (EACA) and tranexamic acid (TE) inhibited tPA-induced plasma clot fibrinolysis with an apparent IC50 of ∼80 μM and ∼20 μM, respectively. Further, efficacy of truncated KD1-L17R was tested in a mouse liver laceration model of bleeding. As compared to saline, the amount of blood loss was reduced by ∼65% by truncated KD1-L17R (N=6, p 0.001), ∼70% by BPTI (N=10, p 0.003), ∼52% by TE (N=10, p 0.019) and ∼25% by EACA (N=16, p 0.03). We also observed seizures in four (25%) of the animals treated with a single dose of EACA.
In conclusion, truncated KD1-L17R is an effective antifibrinolytic agent similar to the 73-residue KD1-L17R and BPTI/Aprotinin. Although lysine analogues are relatively effective in reducing blood loss, EACA caused seizures in our studies. These observations are consistent with recent reports that one of the major side effects of lysine analogues is seizures (Martin et al., J Cardiothorac Vasc Anesth 25, 20–25, 2011; Koster and Schirmer, Curr Opin Anaesthesiol 24, 92–97, 2011). We conclude that truncated KD1-L17R may serve as an excellent alternative to BPTI and lysine analogues in preventing blood loss during major surgeries including coronary artery bypass graft (CABG) surgery. We are currently expressing the 60-residue KD1-L17R (NH2Asn-Ala-Glu······Ile-Glu-Lys) protein for further efficacy studies. We are also generating additional mutant(s) on the 60-residue KD1-L17R molecule for achieving increased plasmin potency without provoking anticoagulant functions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal