Abstract
Abstract 833
HLA-identical siblings (MRD) or 10 of 10 HLA - A, B, C, DRB1 and DQB1 matched unrelated volunteers (MUD) are considered optimal donors for patients who may benefit from allogeneic hematopoietic cell transplantation (allo-HCT). However, many patients, particularly those from ethnic minorities and mixed race backgrounds, will lack such donors.
HLA-haploidentical (Haplo) relatives are available for virtually all patients. Traditional approaches to allo-HCT using Haplo donors have entailed stringent ex-vivo T-cell depletion and intense preparative regimens. Such transplants have been associated with slow immune reconstitution and a high-risk of opportunistic infections and non-relapse mortality (NRM). Recently, another approach to haploidentical transplantation using T-replete grafts and post-transplant cyclophosphamide (Cy) to prevent graft versus host disease (GVHD) and rejection has demonstrated promising results (Brunstein et al Blood 2011, vol118, p 282).
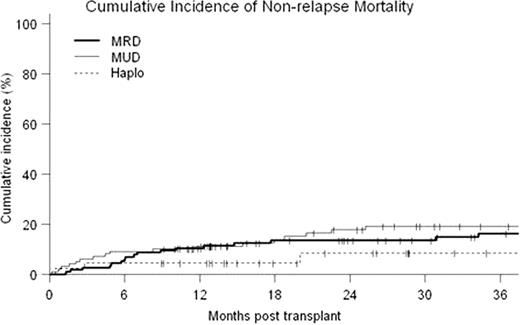
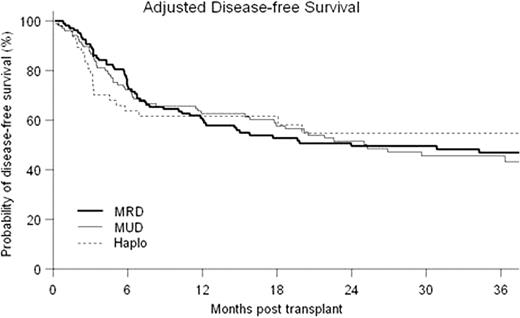
Forty-Five patients underwent first allografts with a Haplo donor at our center between 02/05 and 08/10 (median age=46, range 20–73; diagnoses were AML 14, ALL 6, CML 4, MDS 4, CLL 7, NHL 4, HD 6; median KPS 90, range 70–90; CIBMTR disease risk score low 14, intermediate 7, high 24; median hematopoietic cell transplant co-morbidity index (HCTCI) score = 1, range 0–5; prior autotransplant = 7). Twenty-seven patients received a reduced-intensity conditioning regimen (RIC): fludarabine 30 mg/m2/d d −6 to −2; TBI 200cGy d-1, Cy 14.5 mg/kg/d on d-6,-5 and 50mg/kg/d on d+3,+4 with a bone marrow graft. Eighteen patients received myeloablative conditioning where the above regimen was modified by replacing low-dose TBI with iv busulfan 110–130 mg/m2/d × 4 days and bone marrow was replaced with G-CSF mobilized PBSC as the graft. Two patients receiving RIC failed to engraft and developed autologous hematopoietic recovery. All other patients engrafted with median 100% donor T-cell chimerism from initial testing at d+30. Outcomes of the Haplo transplants were compared to contemporaneous first allografts from MRD (n=115) and MUD (n=99) at our center. Median follow-up for surviving patients was 36 months (range 9–74.5 months). Cumulative probability of NRM at 36 months was 16%, 19% and 8% for MRD, MUD and Haplo respectively (p=NS) (see Fig). For both overall survival (OS) and disease-free survival (DFS), the effects of patient and disease characteristics were evaluated in Cox models with three transplant groups as strata. HCTCI score, CIBMTR risk category, type of diagnosis were identified as significant covariates on both OS and DFS, while age was significant on DFS only. The adjusted probabilities of OS and DFS were estimated from the stratified Cox models, weighted by the proportions of the significant covariates in the pooled sample. MRD had statistically superior OS to Haplo at 18 months (79% vs. 61%, p=0.033 in point wise comparison). However, adjusted OS was not significantly different between the three types of transplant at 36 months (71%, 58% and 58% for MRD, MUD and Haplo respectively (p=NS)). Adjusted DFS at 36 months was 47%, 46% and 55% respectively (p=NS) (see Fig). Ninety-three patients (35.7%) suffered relapse or progression of their malignancy at a median of 154 days post transplant (range 12–1445 days). Estimated cumulative incidence of relapse at 36 months was 35%, 38% and 36% respectively for MRD, MUD and Haplo (p=NS) (see Fig). The inverse probability of censoring weighted (IPCW) method was used to determine survival following relapse/progression in each group. Estimated survival at 1 year following relapse was 71%, 64% and 15% (p<0.0001 pointwise comparison for MRD vs. Haplo and MUD vs. Haplo).
Bashey:Otsuka America Pharmaceuticals, Inc: Research Funding. Sizemore:Otsuka America Pharmaceuticals, Inc: Research Funding. Manion:Otsuka America Pharmaceuticals, Inc: Research Funding. Brown:Otsuka America Pharmaceuticals, Inc: Research Funding. Holland:Otsuka America Pharmaceuticals, Inc.: Research Funding. Solomon:Otsuka America Pharmaceuticals, Inc: Research Funding. Morris:Otsuka America Pharmaceuticals, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal