Abstract
Abstract 82
Plerixafor is thought to enhance chemotherapy mediated myeloid leukemia cell death by promoting mobilization from the protective bone marrow niche and disrupting CXCL12-mediated survival signals. We conducted a Phase 1, open-label, multi-center, dose escalation study in patients (pts) with newly diagnosed AML to determine the maximum tolerated dose (MTD) and safety of plerixafor when combined with cytarabine and daunorubicin.
Adult pts ≤70 years old with newly diagnosed AML were eligible to participate in this ongoing study. Pts with M3 AML and those <50 years old with core binding factor leukemias were excluded. Pts received IV cytarabine 100 mg/m2/day by continuous infusion on days 1–7 and IV daunorubicin 90 mg/m2/day on days 1–3 (7+3 regimen). Plerixafor was given as a 30-min IV infusion, 4–5 hours before daunorubicin beginning on day 2, and repeated at the same time on days 3–7, starting at 0.24 mg/kg, proceeding to dose levels of 0.32, 0.40, and 0.48 mg/kg. Three to 12 evaluable pts were enrolled in each cohort in a modified 3+3 design. Pts were observed for dose-limiting toxicities (DLT) from the first plerixafor dose through day 49. Responses were assessed using IWG response criteria. Serial peripheral blood (PB) samples were analyzed before and after the first and last plerixafor doses for circulating CD34+/CD117+ leukemia cells.
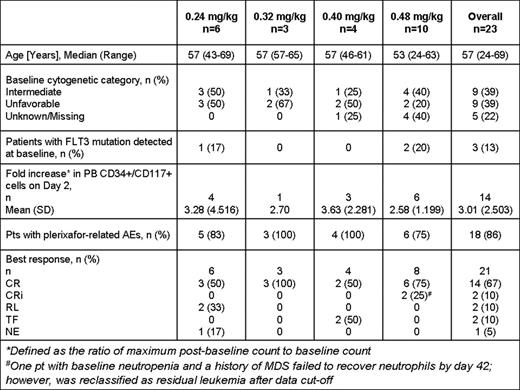
Twenty-three pts (median age 57 years) have been enrolled in 4 cohorts. Baseline characteristics are included in Table 1.
Plerixafor infusion on day 2 caused a rise in PB AML blasts (mean 3.01-fold increase) peaking at 2–4 hours after administration. On day 7, there was a mean 1.51-fold increase in PB AML blasts but far fewer total cells were detected.
Eighteen (86%) pts experienced adverse events (AEs) that were reported as at least possibly related to plerixafor. The majority were Grade 1/2 in severity and mainly included gastrointestinal disorders. Four (19%) pts experienced Grade 3 plerixafor-related AEs including febrile neutropenia (n=3), neutropenia (n=1), nausea (n=1), infections (n=2) and decreased appetite (n=1) commonly observed with 7+3 regimen. One (5%) pt (0.48 mg/kg cohort) experienced Grade 4 related AEs of thrombocytopenia and asymptomatic pulmonary embolism (while receiving medroxyprogesterone); the latter was the only possibly-related SAE reported. The median time to neutrophil (≥ 0.5 × 109/L) and platelet (≥100 × 109/L) recovery for responders was 19.5 (range 13–35) and 21 (range 17–37) days, respectively. There were 4 (17%) plerixafor unrelated deaths (0.24mg/kg): 1 within 30 days post induction due to an AE of acute respiratory distress syndrome and 3 due to disease progression > 3 months post induction. No DLTs have been reported.
Of 21 pts with available data, 14 (67%) had complete response (CR), 2 had CR with incomplete count recovery (CRi), 2 had residual leukemia (RL), 2 had treatment failure (TF) due to resistant disease and 1 was not evaluable (NE) due to early death.
The toxicity or hematopoietic recovery expected with the 7+3 regimen was not significantly altered by the addition of plerixafor. Transient mobilization of AML blasts into PB was observed immediately following plerixafor treatment. Sixteen of 21 patients, majority of who had intermediate or poor risk cytogenetics, achieved a CR or CRi, with responses observed across all plerixafor doses. Twice daily plerixafor dosing and addition of G-CSF to augment mobilization are being currently explored.
Uy:Sanofi Oncology: Consultancy, Speakers Bureau. Off Label Use: Plerixafor (Mozobil®), a hematopoietic stem cell mobilizer, is approved by the US FDA in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma. Avigan:Sanofi Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Ariad: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chemgenex: Membership on an entity's Board of Directors or advisory committees, Research Funding. Becker:Millenium: Research Funding; Glycomimetics: Research Funding; Sanofi Oncology: Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Consultancy. Hewes:Sanofi Oncology: Employment. Johns:Sanofi Oncology: Employment. Erba:Ambit: Research Funding; Ascenta: Research Funding; Celgene: Speakers Bureau; Chroma: Research Funding; Eli Lilly: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal