Abstract
The iron chelators in current clinical use - deferoxamine, deferasirox, deferiprone - are useful for many but not all patients with transfusion-dependent anemias. FBS0701 is a novel, orally available tridentate iron chelator in clinical development. Pre-clinical studies demonstrated a >4-fold higher no-observable-adverse-effect level compared to deferasirox (Exjade®) suggesting a favorable clinical safety profile, especially with respect to gastrointestinal and renal toxicity. Multi-dose safety and PK studies in transfusional iron-overloaded patients established the acute safety of FBS0701 and support once-a-day dosing.
To assess the long term safety, tolerability, dose-response and pharmacodynamics of FBS0701 in adults with transfusional iron overload.
This was a 24 week, multi-center study of FBS0701 at two once-daily dose levels: 14.5 (16 mg of the salt form) and 29 (32 mg salt) mg/kg/d. These doses are the molar equivalent of 13.5 and 27 mg/kg of deferasirox. Adults under age 60 y, with liver iron concentration (LIC) measured by R2 (Ferriscan®) MRI at baseline between 3.5 and 30 mg/g dry weight and cardiac T2* >10 ms were eligible for enrolment. Fifty-one patients were stratified on the basis of historical mean daily transfusion iron intake and then randomized to dose. Patients were evaluated at Weeks 1, 2, 3, 4, 6, 8 and then monthly to 24 wks. Assessments included history and physical exam, vital signs, clinical pathology, adverse events (AEs), ECG and LIC by R2 MRI at baseline, 12 and 24 weeks. All results shown are from the safety population (patients who received at least one dose FBS0701)
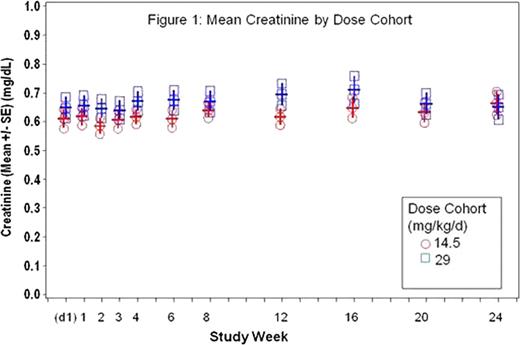
All patients had a primary hemoglobinopathy, the majority (75%) with β-thalassemia syndromes. Twenty-four (47%) patients had abnormal transaminases prior to dosing and 16 patients (31%) had a history of viral hepatitis. FBS0701 was generally well tolerated at both doses. Forty-nine patients (96%) completed the study; two withdrew for reasons unrelated to drug. There were no serious AEs related to FBS0701. No AEs showed dose-dependency in frequency or severity. Fewer than 5% had nausea, vomiting, abdominal pain, or diarrhea deemed related to FBS0701. The most common AE deemed related to FBS0701 was an increase in transaminases (15%, N=8). Three of these patients acquired HCV on-study from a single blood bank source. None of the remaining patients exceeded their baseline transaminases by 5X. Mean serum creatinine (Cr) in the two dose groups were not statistically different from each other or from baseline values (Figure 1). Only three (5%) patients, each with GFR <100 mL/min at baseline, had an abnormal Cr on-study and none exceeded 50% above ULN. All changes in Cr were reversible. At Week 24 there was a statistically difference between the two doses in the change in LIC compared to baseline (Figure 2). The low dose cohort had a mean LIC gain of 3.1 mg/g (dw), with 29% achieving a net decrease in LIC in the intent to treat population. The high dose cohort (29 mg/kg) had a mean change of −0.3 mg/g (dw), with 50% achieving a net decrease in LIC over 24 weeks. (P value <0.04 for the difference in mean LIC).
FBS0701 for 24 weeks was equally well tolerated at both doses: AEs related to drug were each <5% - nausea, vomiting, abdominal pain, or diarrhea. Cr was stable in 95% of patients even in the majority with moderate renal impairment (GFR <100 mL/min) at baseline. Transaminases were elevated in 15% and reversible. Patients with a history of hepatitis were more likely to have elevated transaminases (80%) compared to patients without (20%), but there were no differences in the magnitude or duration of elevations between the two groups. The low frequency, short duration and generally mild nature of AEs at therapeutic doses compares favorably to other oral iron chelators. FBS0701 was also generally well tolerated in patients with common co-morbidities including infectious hepatitis and renal impairment. FBS0701 was effective at clearing hepatic iron in a majority of patients receiving 29 mg/kg/d. Higher doses of FBS0701 might be expected to safely induce negative iron balance in a greater proportion of iron overloaded patients.
Neufeld:Ferrokin BioSciences: Research Funding; Novartis: Research Funding. Galanello:Apopharma: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Ferrokin Biosciences: Research Funding. Viprakasit:Ferrokin BioSciences: Research Funding; Novartis: Research Funding, Speakers Bureau; GPO-L-ONE: Research Funding; National Research University, Thailand: Research Funding. Aydinok:Ferrokin Biosciences: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau. Piga:Ferrokin BioSciences: Research Funding. Harmatz:Novartis: Research Funding. Forni:Ferrokin BioSciences: Research Funding; Fondazione Carige, Genoa, IT: Research Funding; Novartis: Research Funding, Speakers Bureau. Shah:Swedish Orphan Biovitrum: Speakers Bureau; Novartis: Speakers Bureau; Ferrokin Biosciences: Research Funding. Grace:Ferrokin BioSciences: Research Funding. Porter:Ferrokin BioSciences: Research Funding. Vichinsky:Apopharma: Research Funding; Ferrokin Biosciences: Research Funding; Novartis: Research Funding. Wood:ApoPharma: Consultancy; Novar: Research Funding; Ferrok: Consultancy. Peppe:Ferrokin BioSciences: Employment. Jones:Ferrokin BioSciences: Employment. Rienhoff:Ferrokin BioSciences: Employment, Equity Ownership.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal