Abstract
Abstract 663
The majority of patients with relapsed or refractory B-cell, non-Hodgkin's lymphoma (NHL) are over 60 years of age, yet many are denied potentially curative high-dose regimens due to concerns of excessive toxicity. We have shown that myeloablative anti-CD20 radioimmunotherapy (RIT) can safely deliver effective radiation doses to tumor sites while limiting exposure to normal organs in older adults requiring high-dose therapy, however, not all patients in this series remained progression-free (Gopal, JCO 2007). Preclinical data suggest that improved anti-tumor activity may be attained by the concurrent administration of nucleoside analogs (fludarabine, cytarabine) which synergize with RIT (Johnson, Int J Cancer; Gopal, BBMT, 2006). We hypothesized that a prolonged regimen of fludarabine could be administered concurrently with myeloablative RIT and ASCT to safely augment the efficacy of this approach. We present the phase I data evaluating this strategy.
Patients were eligible if they were 60 years of age or older, had mantle cell lymphoma (MCL) in first remission or relapsed/refractory B-NHL, an ECOG PS of 0–1, acceptable organ function, >2×106 autologous CD34+ peripheral blood stem cells/kg collected, <20 Gy prior radiation to critical organs, and no human-anti-mouse-antibodies (HAMA). All patients underwent outpatient biodistribution studies for dosimetry using tositumomab (1.7 mg/kg, n=3 or 485mg flat dose, n=33) labeled with ∼10mCi I-131 followed by serial quantitative gamma camera imaging to calculate individualized organ-specific absorbed dose estimates. Patients then received therapeutic infusions of I-131-tositumomab to deliver 27Gy to the critical normal organ receiving the highest radiation exposure. Forty-eight hours later fludarabine was administered in escalating doses to patients (Table) to define a regimen associated with a dose limiting toxicity (DLT = grade III/IV Bearman toxicity) rate of <25%. ASCT occurred when radiation exposure was estimated to be <2mR/hr at 1meter. Filgrastim at 5μg/kg/day or pegfilgrastim at 6mg × 1 was started on day 1. Response was scored using standard criteria.
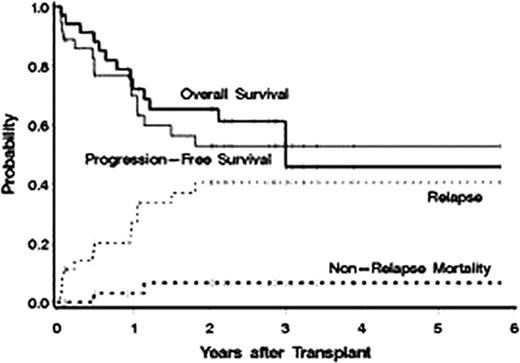
Between July 2005 and May 2011 36 patients were treated. Baseline characteristics included: median age 65 yrs (range 60–76), stage III/IV = 34 (94%), median number of prior regimens = 2 (range 1–9), chemoresistant disease (defined as < a partial response [PR] to the most recent regimen) = 12 (33%), >1 extranodal site = 14 (39%), elevated LDH at treatment = 13 (36%), IPI score at transplant 3–5 = 53%. Histology: MCL = 23, diffuse large B-cell = 8 (with 5 transformed from follicular lymphoma [FL]), FL=3, and marginal zone = 1, Waldenstrom's = 1. Dose limiting organs receiving up to 27Gy included lung (30), kidney (4), and liver (2) with a median administered I-131 activity of 471 mCi (range 260–1620). Fludarabine was escalated from 10 mg/m2/day × 5 days to 30 mg/m2 × 7 days without observation of a DLT (Table). The median CD34 dose was 5.42 ×106/kg with neutrophil (ANC>500μl) and platelet (>20 K/μl) engraftment occurring a median on 10 and 12 days after ASCT, respectively. Only 2 patients developed grade 4 NCI-CTC grade 4 non-hematologic toxicities (hypokalemia/hypophosphatemia, depression), 25 (69%) remained outpatients after discharge from radiation isolation, and there were no non-relapse deaths in the first 100 days after transplant. Responses to therapy were as follows: CR/CRu = 26 (79%), PR = 2 (6%), SD = 4 (11%), and PD = 4 (11%). Currently, 23 (64%) patients are alive with 22 (61%) progression free. The estimated 3 yr overall survival, progression-free survival, relapse, and non-relapse mortality are 54%, 53%, 41%, and 7%, respectively (median follow up of 2.5yrs after ASCT, Figure). Improved survival was associated with <2 prior regimens (HR=.08, p=.02) and chemosensitive disease (HR=.35, p=.07).
Dose escalation schema and dose limiting toxicities (DLT) of High-dose (27Gy) I-131 tositumomab + fludarabine + ASCT
| Dose level . | n . | Fludarabine daily dose (mg/m2) . | # of doses . | Total dose (mg/m2) . | # DLT . |
|---|---|---|---|---|---|
| 0 | 7 | 10 | 5 | 50 | 0 |
| 1 | 5 | 15 | 5 | 75 | 0 |
| 2 | 5 | 20 | 5 | 100 | 0 |
| 3 | 5 | 25 | 5 | 125 | 0 |
| 4 | 6 | 30 | 5 | 150 | 0 |
| 5 | 5 | 30 | 6 | 180 | 0 |
| 6 | 3 | 30 | 7 | 210 | 0 |
| Dose level . | n . | Fludarabine daily dose (mg/m2) . | # of doses . | Total dose (mg/m2) . | # DLT . |
|---|---|---|---|---|---|
| 0 | 7 | 10 | 5 | 50 | 0 |
| 1 | 5 | 15 | 5 | 75 | 0 |
| 2 | 5 | 20 | 5 | 100 | 0 |
| 3 | 5 | 25 | 5 | 125 | 0 |
| 4 | 6 | 30 | 5 | 150 | 0 |
| 5 | 5 | 30 | 6 | 180 | 0 |
| 6 | 3 | 30 | 7 | 210 | 0 |
High-dose (27 Gy) I-131 tositumomab can safely be administered concurrently with up to 210 mg/m2 fludarabine in an older, high-risk patient population. This strategy warrants further investigation as a method to safely augment the antitumor activity of myeloablative RIT.
Gopal:GSK: Research Funding; Spectrum: Research Funding; Seattle Genetics: Consultancy, Research Funding; Merck: Research Funding; Piramal: Research Funding; Cephalon: Research Funding; Millenium: Speakers Bureau; Abbott: Research Funding; Pfizer: Research Funding; SBio: Research Funding; Bio Marin: Research Funding; Biogen-Idec: Research Funding. Off Label Use: High-dose use of I-131-tositumomab. Maloney:GSK: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal