Abstract
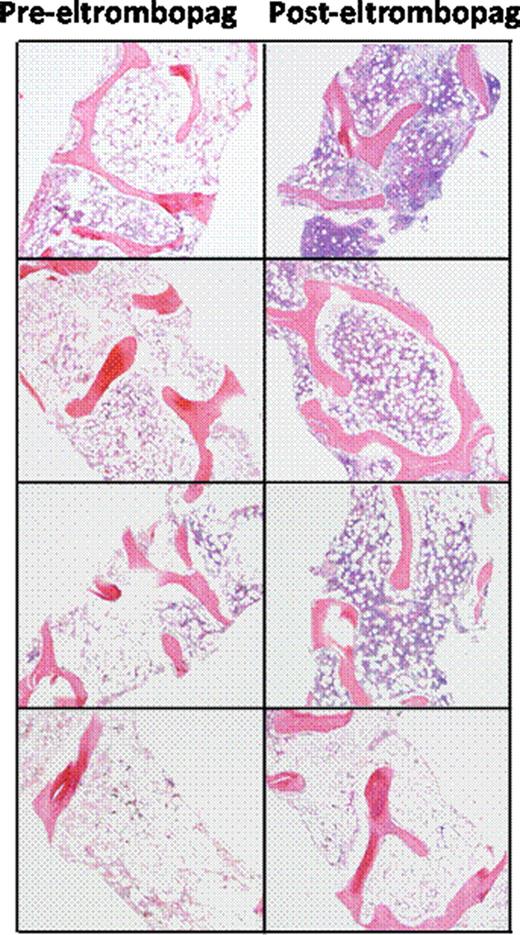
Severe aplastic anemia (SAA) is characterized by trilineage marrow hypoplasia and a paucity of hematopoietic stem cell (HSC) progenitors. SAA is treated with immunosuppressive therapy (IST) or allogeneic HSC transplantation (HSCT), with a successful outcome after either treatment in a majority of patients. However, 20–40% of patients without a suitable donor for HSCT and a suboptimal response to IST may have persistent severe thrombocytopenia. Thrombopoietin (TPO) is the principal regulator of platelet production, and it exerts its effects through binding the megakaryocyte progenitor TPO receptor mpl, which stimulates production of mature megakaryocytes and platelets. Several lines of evidence support the concept that signaling through mpl also influences expansion and maintenance of primitive HSCs and multi-potent progenitor cells. Eltrombopag, is a small molecule TPO receptor agonist that stimulates mpl, and increases platelet counts in patients with chronic immune thrombocytopenic purpura (ITP). It is approved for the treatment of chronic ITP (Promacta®). We conducted a non-randomized, pilot phase II study of eltrombopag in SAA patients who remained severely thrombocytopenic at least six months after one or more rounds of IST (clinical trials.gov identifier NCT00922883). Consecutive patients fulfilling the inclusion criteria received eltrombopag 50mg daily with dose escalation every two weeks to a maximum dose of 150mg daily. Primary end points assessed after three months of treatment were changes in peripheral blood counts (platelets, hemoglobin, absolute neutrophil counts), with hematologic response criteria defined a priori for each lineage. Secondary endpoints included the incidence of bleeding events, and health related quality of life. Patients who achieved hematologic responses were maintained on eltrombopag through an extended access protocol. We completed our planned accrual of twenty five patients, and 22 are evaluable for response to date. Median age was 45 years old (range 18–77 years), and the median time from the last course of IST was 13 months (range 6–54 months). Median follow up time was 9 months (range 1–24 months). Nine of twenty two patients (41%) achieved hematologic responses: seven of twenty-two patients (32%) achieved platelet responses with transfusion independence for eight weeks or greater; six patients had improved hemoglobin levels after starting treatment (mean hemoglobin increase of 3.8 g/dL) and 4 patients who were previously dependent on red blood cell transfusions have achieved transfusion-independence. Five neutropenic patients had increased neutrophil counts after treatment with eltrombopag (mean increase 660 cells/uL). Plasma TPO levels did not predict for hematologic response to eltrombopag. Serial bone marrow biopsies performed on patients with hematologic responses demonstrated normalization of trilineage hematopoiesis and cellularity in three of four responders receiving a year or more of therapy, with no increase in reticulin fibrosis (Figure 1). These results represent the first evidence that TPO stimulation can expand the HSC pool in humans, with clinically meaningful trilineage hematologic improvements in patients with SAA, resulting in transfusion-independence and improved quality of life with a simple daily oral regimen. Updated response data on the full 25 patients will be presented at the Society's meeting.
Disclosures:
Off Label Use: Eltrombopag for severe aplastic anemia.

This icon denotes a clinically relevant abstract
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal