Abstract
Abstract 5150
Essential thrombocythemia (ET), polycythemia vera (PV) and myelofibrosis (MF) are myeloproliferative disorders (MPDs) characterized by a chronic over-production of cells of one or more blood cell lineages and/or bone marrow fibrosis which may, on occasion, progress to acute myeloid leukemia. The V617F gain of function mutation in the pseudokinase domain of Jak2, which results in constitutive activation of Jak2, is the most frequent mutation associated with MPD. Constitutively activated Jak2 can lead to dysregulated downstream signaling pathways (STAT, MAP kinase, and PI3 kinase) which in turn trigger abnormal growth, survival and differentiation of hematopoietic progenitors. Therefore, inhibition of constitutively activated Jak2 may offer therapeutic potential. Designing a Jak2V617F specific inhibitor encounters challenges due to the lack of enzymatic activity of the pseudokinase domain of Jak2. In lieu of a Jak2V617F mutant selective inhibitor, a highly selective inhibitor of Jak2 is likely an attainable goal.
Jak2 is a member of the Jak family of kinases including Jak1, Jak3, and Tyk2. Highly selective Jak2 inhibitors may provide a better safety margin in chronic dosing settings in ET and PV patients since inhibiting other Jak family members could cause side-effects such as immunosuppression. Attaining the desired selectivity of Jak2 inhibition versus the other family members has been challenging and few compounds have been reported to date that have the desired Jak2 selectivity. This can be attributed to the high homology of the ATP binding pocket among Jak family members, but is also hampered by a lack of assays capable of distinguishing the Jak-selectivity profile in a physiologically relevant setting.
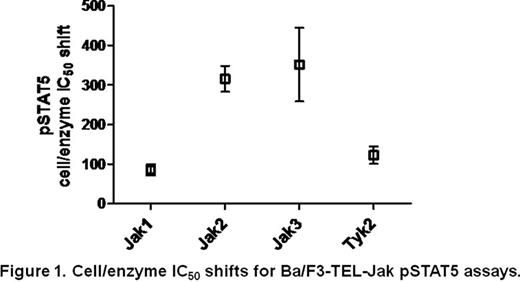
We also compared the potency and selectivity of compounds in the isogenic Ba/F3-TEL-Jak cell lines with data obtained with cytokine stimulated peripheral blood mononuclear cells (PBMCs). The potency and selectivity of compounds in PBMCs are determined by measuring the inhibition of phosphorylation of STAT5 in TPO or GM-CSF stimulated platelets or monocytes (mediated by Jak2) and in IL-2 stimulated lymphocytes (mediated by Jak1 and Jak3). We found that potency correlated well between PBMCs and Ba/F3-TEL-Jak2 cells, and the rank order of compounds based on IC50 values obtained with Ba/F3-TEL-Jak cell lines were conserved well in PBMCs; the compound selectivity profiles derived from the Ba/F3-TEL-Jak cell assays were predictive of Jak2 selectivity profiles obtained in the PBMC assays. Therefore, inclusion of Ba/F3-TEL-Jak pSTAT5 cellular assays may be useful for Jak family inhibitor development. Our results also suggest that relying solely on enzyme potency and selectivity data can be misleading, and that evaluating cellular selectivity in a biologically relevant context may provide a more meaningful understanding of selectivity and lead to the development of more selective Jak2 compounds.
Liu:Amgen, Inc: Employment. Yu:Amgen: Employment. Pistillo:Amgen: Employment. Lee:Amgen: Employment. Schenkel:Amgen: Employment. Geuns-Meyer:Amgen: Employment. Archibeque:Amgen: Employment. Sinclair:Amgen: Employment. Emkey:Amgen: Employment. Molineux:Amgen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal