Abstract
Abstract 5146
Myelofibrosis (MF) is characterized by splenomegaly, debilitating symptoms, hematologic abnormalities, and shortened survival. COMFORT-I is a Phase III randomized, double-blind, placebo-controlled study of the JAK1 and JAK2 inhibitor ruxolitinib in patients (pts) with MF. The placebo arm of this ongoing study provides a unique population in which to assess the clinical burden of MF in a controlled setting.
151 pts with intermediate-2 or high-risk primary-MF (PMF), post-polycythemia vera-MF (PPV-MF), or post-essential thombocythemia-MF (PET-MF) who were not candidates for other therapies were randomized to receive placebo in COMFORT-I after a 28-day washout of previous MF therapies. Assessments included MF symptoms measured daily using an electronic diary (modified Myelofibrosis Symptom Assessment Form [MFSAF] v2.0), spleen volume measured every 12 wks by blinded abdominal MRI or CT scans, and laboratory measures at least monthly through wk 24. The Patient-Reported Outcomes Measurement System (PROMISE) Fatigue and Patient Global Impression of Change (PGIC) questionnaires were administered at each visit. Pts with missing data (baseline or post-baseline assessment measured), or pts who withdrew or crossed over to active treatment before a study visit were not included in analyses.
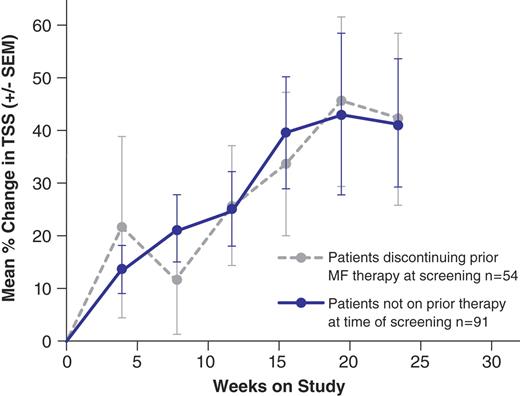
151 pts received 1 or more doses of placebo (median exposure 204 days). The study included 65% high-risk and 35% intermediate-2 risk pts, with a median of 2.5 years since diagnosis. 55% had PMF, 31% PPV-MF, and 14% PET-MF. Regardless of prior MF therapy (57% were on hydroxyurea), there was continual worsening of symptoms over time as assessed by the MFSAF Total Symptom Score (TSS, comprised of scores for night sweats, itching, abdominal pain and discomfort, early satiety and bone/muscle pain) (see Figure).
At wk 24, mean/median spleen volume increased by +8.1%/+8.5%, and both intermediate-2 and high-risk pts demonstrated progressive disease over the course of the study. Mean/median PROMIS Fatigue score worsened by 9.1%/6.5%. Mean/median score for the PGIC (a 7-point scale ranging from 1 = very much improved to 7 = very much worse, with 4 = no change) was 4.2/4.0 at wk 24; 19.6% of pts reported feeling much worse or very much worse. Mean/median total body weight changed from 72.0 kg/72.2 kg to 70.1 kg/69.7 kg at wk 24. Hematology assessments (median, baseline vs wk 24) were as follows: hemoglobin, 105 vs 106 g/L; platelets, 233 vs 219 x109/L; neutrophils, 14.8 vs 16.0 x109/L; white blood cell count, 18.4 vs 20.1 x109/L. Two of 151 pts had new-onset (new or worsening) grade 3 or grade 4 thrombocytopenia, and 23/151 pts had new-onset grade 3 or grade 4 anemia. 16 pts met protocol-specified criteria for unblinding and crossover to active treatment before wk 24, which was a 25% increase in MRI-based spleen volume either with or without increased pain or weight loss; thus, mean and median values reported at wk 24 may represent an underestimate of the degree of worsening in disease parameters.
The signs and symptoms of MF continued to worsen over a 6-month time frame in this placebo population. The data illustrate the progressive nature of MF and support the need for effective therapeutic interventions. Moreover, treatment benefit must be benchmarked against continual evolution of the disease in the absence of treatment. Early recognition of the debilitating symptoms of MF and intervention with effective therapy to prevent continual worsening of the disease should be strongly considered.
Verstovsek:Incyte: Research Funding. Mesa:Incyte: Research Funding; Lilly: Research Funding; SBio: Research Funding; Astra Zeneca: Research Funding; NS Pharma: Research Funding; Celgene: Research Funding. Gotlib:Incyte: Consultancy, Research Funding. Levy:Incyte: Employment, Equity Ownership. Gupta:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Catalano:Incyte: Honoraria; Novartis: Honoraria. Deininger:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Genzyme: Research Funding. Miller:Incyte: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau. Winton:Incyte: Consultancy. Arcasoy:Incyte: Research Funding. Lyons:Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Telik: Research Funding; Alexion: Consultancy, Honoraria; Novartis: Research Funding. Vaddi:Incyte: Employment. Erickson-Viitanen:Incyte: Employment. Sun:Incyte: Employment. Sandor:Incyte: Employment. Kantarjian:Incyte: Research Funding; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal