Abstract
Abstract 4782
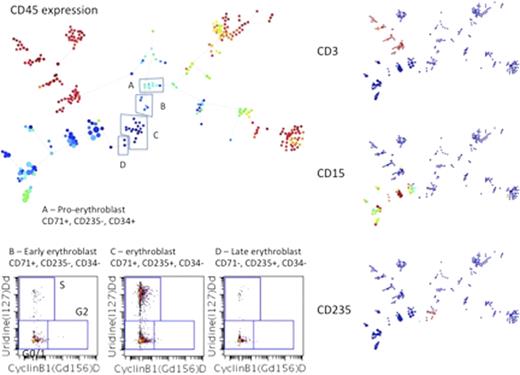
Mass cytometry is a recently introduced proteomic technology that permits simultaneous measurements currently of as many as 40 parameters per single cell, including surface markers and intracellular signaling molecules and has recently provided a system-wide view of immune/hematopoietic cell differentiation within human bone marrow (Bendall et al, Science 2011). Given the unprecedented level of detail at the single cell level this approach provides, this technology is ideal for delineating the molecular and cellular basis of tumor heterogeneity. As an extension from our recent mass cytometry studies, we have now developed a method to perform cell cycle analysis by utilizing uridine incorporation, simultaneously with determinations of other cell cycle phase markers such as cyclins A, B1, and D as well as phosphorylation of retinoblastoma protein, histone H3, plus markers of apoptosis and other critical markers that demarcate malignant dysregulation. This allows for cell cycle state characterization that can be employed in both cell lines and primary cells growing in vitro or in vivo. We have combined this new method with a hierarchical clustering algorithm (SPADE) to profile the cell cycle state distribution across the continuum of hematopoietic differentiation in normal bone marrow. We observe the expected quiescence of terminally differentiated cells, hematopoietic progenitor and stem cell populations, and note that the majority of cell proliferation is confined to immunophenotypically immature cell populations of the myeloid, erythroid and monocytic linages. We have begun a broad comparison of these “normal” proliferation states to those observed across punctuated states of malignant progression that vary in “differentiation” of malignant cells in primary human sample in patients with acute myeloid leukemia revealing. The distribution of proliferation across these subpopulations generally parallels that of normal samples with more immunophenotypically “differentiated” AML cell populations having lower proliferative rates, however, not all AML patient samples follow this trend.
Disclosures:
Fantl:Nodality, Inc.: Equity Ownership.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal