Abstract
Abstract 4160
Allogeneic stem cell transplantation (allo-SCT) remains the only curative therapy for patients with indolent B cell Non-Hodgkin's Lymphoma (B-NHL) as well as patients with aggressive B-NHL that have failed prior autologous stem cell transplants (ASCT). Myeloablative conditioning is associated with a high incidence of early transplant related mortality (TRM) particularly in patients with extensive prior therapy and advanced age. Non-myeloablative conditioning (NMA) provides less TRM and the opportunity to exploit graft-versus-lymphoma (GVL) effects at the expense of conditioning intensity for disease control. The addition of rituximab to these regimens provides both anti-lymphoma and potential immunomodulatory benefit in terms of decreasing the incidence of graft-versus-host disease (GVHD). The aim of this phase 2 trial was to determine the safety and efficacy of low-dose total body irradiation (TBI), cyclophosphamide, fludarabine and peri-transplant rituximab in patients with CD20+ B cell malignancies.
This analysis includes 35 B-NHL patients with a median age of 54 years (range 33–67) at the time of allo-SCT. Diagnoses included: 15 chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), 9 follicular lymphoma (FL), 6 diffuse large B cell lymphoma (DLBCL)/ transformed lymphoma (B-tNHL), 3 mantle cell lymphoma (MCL) and 2 other. Patients were previously treated with a median of 3 prior chemotherapy regimens (range 1–5) and 6 patients had a previous ASCT. Twenty-five patients were chemosensitive at the time of allo-SCT by International Criteria (Cheson et al JCO 1999, Hallek et al Blood 2008 for CLL/SLL). Patients with DLBCL, B-tNHL and MCL were required to have chemosensitivity prior to allo-SCT. Patients with FL and CLL/SLL were not required to achieve chemosensitivity, but did require stable disease, prior to allo-SCT. Conditioning consisted of cyclophosphamide 50 mg/kg, fludarabine 25 mg/m2 daily over 5 days and 200 cGy TBI. Rituximab 375 mg/m2 was administered 1–2 days prior to the initiation of conditioning and weekly for 4 doses beginning d+21. Graft rejection prophylaxis with equine anti-thymocyte globulin (ATG), 30 mg/kg daily d-3 and d-2, was given to recipients of unrelated grafts. All patients received a G-CSF mobilized peripheral blood stem cell graft. GVHD prophylaxis consisted of tacrolimus and sirolimus beginning d-3 and methotrexate 5 mg/m2on d+1, d+3 and d+6. Twelve patients received a graft from a 10/10 HLA matched related donor, 19 from a 10/10 HLA matched unrelated donor and 4 from a 9/10 HLA mismatched unrelated donor. A survival event was defined as progression of disease (PD) or death from any cause, including transplant-related mortality (TRM).
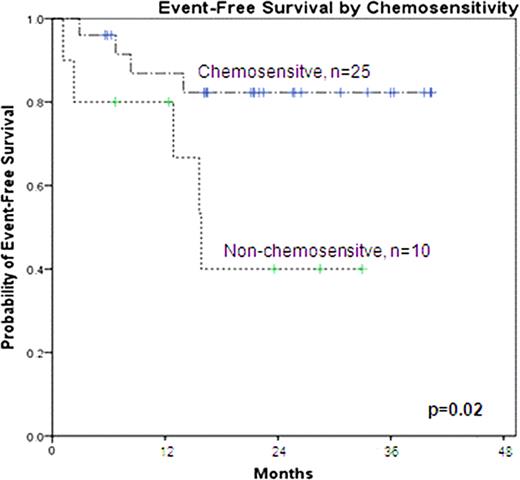
All patients engrafted and full donor chimerism in neutrophils and T cells was documented at 3 months posttransplant in 30 evaluable patients. This regimen was well tolerated and no grade 3–4 oral mucositis was observed. Acute GvHD, grades 2 to 4, developed prior to d+100 in 20% of evaluable patients (8% grade 3–4). The 2-year incidence of chronic GVHD (CIBMTR criteria) in 32 patients at risk was 65% (23% mild, 34% moderate and 9% severe). Twenty six patients are alive with a median of 23 months (range 6–41). Of the 9 events, 6 were TRM (5 from GVHD) and 3 PD (all of which died of lymphoma). No events have occurred beyond 15 months. The 2-year EFS for all patients is 72%. No difference in EFS or OS was demonstrated according to: histology, graft source, number of previous lines of therapy, previous autologous transplant or age. The only variable associated with EFS was pre-allo-SCT chemosensitivity. The 2-year EFS for chemosensitive patients was 83% compared to 44% for patients without chemosensitivity pre-allo-SCT (see figure, p=0.02).
This NMA regimen with peri-transplant rituximab is effective and safe allowing full donor myeloid and T cell chimerism with minimal toxicities and with notable anti-lymphoma effect, however chronic GvHD developed in a significant number of patients. In this trial, peri-transplant rituximab may provide disease control but its role on cGvHD prevention is not clear. These data confirm chemosensitivity as a significant prognostic factor to survival in B-NHL with NMA allo-SCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal