Abstract
Abstract 3995
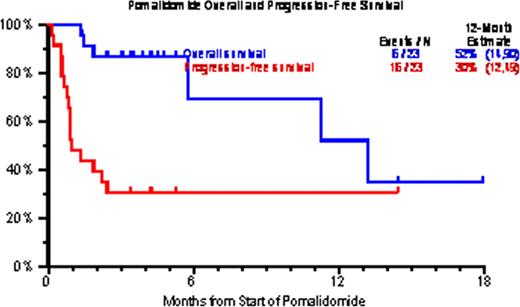
Pom is a third generation immunomodulatory drug (IMiD) which has demonstrated safety and efficacy in RRMM with prior exposure/resistant to other IMiDs and bortezomib. This is the first report on the UARK Pom compassionate use experience in RRMM. Methods: First cycle Pom was given at 4mg orally Days 1–21 every 28 days; dexamethasone (DEX) was given to 9/23 patients at doses varying from 12 to 40 mg on schedules ranging from Days 1–4, weekly, twice a week, or three times a week. In the absence of at least PR, Pom dose was escalated to 5mg. 1patient also received bortezomib and 1 patient received bortezomib and cytoxan. Cox regression modeling was employed for univariate and multivariate analyses, whereas Kaplan-Meier curves were used for overall survival (OS) and progression free survival (PFS). Results: 23 patients with RRMM were enrolled. Baseline characteristics included age >=65yr in 43%, ISS stage >=II was seen in 78% of patients, cytogenetic abnormalities (CA) within 6 months in 80%, and GEP-defined high risk in 41% of patients. 22/23 patients (96%) had prior autologous stem cell transplant. 19/23 patients (83%) had at least 2 transplants. All 23 patients had disease progression after having received regimens containing bortezomib, thalidomide, lenalidomide, melphalan and steroids. At least 1 cycle of treatment was administered to all 23 patients enrolled, 52% of patients received >1 cycle of treatment and only 13% received =>5 cycles. 10 patients (43%) discontinued therapy primarily due to progression or death. 5/23 (22%) patients achieved PR, 57% had stable disease. A trend towards PFS benefit was observed in patients receiving cycle 2 (HR=0.30, p=0.215) on univariate analysis and multivariate analysis (HR=0.40, p=0.48) after adjusting for GEP-defined risk status (HR=2.69, p=0.16). Most common toxicities, counting all toxicities (>=grade 3) were: thrombocytopenia (70%), leukopenia (61%), anemia (43%), hypophosphatemia (35%) and hypokalemia (26%). Overall and progression- free survival at 12 months were 52% and 30%, respectively. Conclusions: Pom demonstrates anti-myeloma activity in this advanced RRM population, especially in a sub-population with GEP-defined low-risk disease.
Barlogie:Celgene: Consultancy, Honoraria, Research Funding; IMF: Consultancy, Honoraria; MMRF: Consultancy; Millennium: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy; Novartis: Research Funding; NCI: Research Funding; Johnson & Johnson: Research Funding; Centocor: Research Funding; Onyx: Research Funding; Icon: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal