Abstract
Abstract 3994
Patients with MM undergoing autologous stem cell transplant (ASCT) achieving complete response (CR) or very good partial response (VGPR) have prolonged progression free survival (PFS) and overall survival (OS) compared to the patients that achieve <VGPR prior to ASCT (Lahuerta JJ et al., 2008; Moreau P et al., 2011). Therefore it is of profound significance to attain the best response with induction therapies to obtain the better long-term outcomes. The response rates have significantly improved since the introduction of bortezomib, a proteasome inhibitor, in the induction therapies for myeloma. We performed a meta-analysis to evaluate the efficacy of the addition of bortezomib to the existing regimens used in induction therapy.
We searched Medline, Embase, Cochrane databases and ASH, ASCO conference proceedings from 01/2000 through 08/2011 for publications and abstracts to identify the phase III RCTs comparing BCIR vs. NBCIR in transplant-eligible patients with myeloma. A meta-analysis was performed using both the fixed (Mantel-Haenszel) and random (DerSimonain and Laird) models to calculate the risk difference with the comparator arm to evaluate the rates of CR, ≥VGPR, ORR, PFS, OS and toxicities. Altogether, we identified 4 RCTs (two published articles and unpublished data from two RCTs including 2086 patients). The consistency of results (effect sizes) among studies was investigated by means of two heterogeneity tests, the χ 2-based Cochran's Q test, and the I2 Statistic. We considered that heterogeneity was present when the P value of the Cochran's Q test was <.1 and I 2 statistic was > 50%.
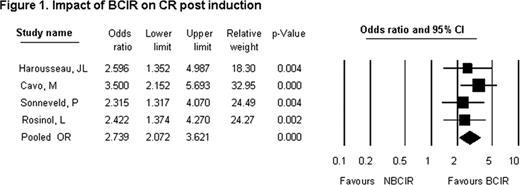
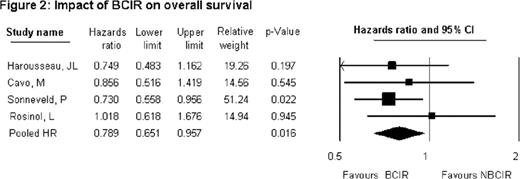
Q-statistic for ORR (P =0.338; df =3; I2 = 11.1); ≥VGPR (P =0.175; df =3; I2 = 39.53); CR (P =0.677; df =3; I2 = 0) suggests homogeneity across studies. Pooled odds ratios of overall response rates (ORR), ≥VGPR, CR with BCIR vs. NBCIR were 2.619 (P <0.000; 95% CI: 2.103–3.261); 3.558 (P <0.000; 95% CI: 2.908–4.354); 2.739 (P <0.000; 95% CI: 2.072–3.621) respectively indicating BCIR result in improved efficacy. Similar results were translated post-ASCT demonstrating the superiority of BCIR over NBCIR. Post-ASCT ORR (p =0.141; df =3; I2 = 45.03); ≥VGPR (P =0.442; df =3; I2 = 0); CR (P =1.00; df =3; I2 = 0) suggest homogeneity. Pooled odds ratios of ORR, ≥VGPR, CR post-ASCT for BCIR vs. NBCIR were 1.907 (P <0.000; 95% CI: 1.431–2.639); 2.43 (P <0.000; 95% CI: 2.025–2.914); 2.406 (P <0.000; 95% CI: 1.966–2.945) respectively. The pooled hazard ratios (HR) for 3 year PFS and OS were HR 0.723 (95% CI 0.620–0.844; P =0.000); 3 year OS HR 0.789 (95% CI 0.651–0.957; P =0.016) respectively in favor of BCIR. The relative risk (RR) of selected ≥grade 3 toxicities was higher with BCIR. RR of peripheral neuropathy (PN) was 2.469 (95% CI 1.848–3.297; P =0.000) and infection with herpes-zoster virus (HZV) was 2.197 (95% CI 1.368–3.529; P =0.001). The RR of a thromboembolic event (TEE) with BCIR was 0.8 (95% CI 0.56–1.15; P =0.206).
Our mixed model meta-analysis demonstrates that the addition of bortezomib to the induction regimens in the transplant-eligible patients with MM results in improved ORR, ≥VGPR, CR, PFS and OS compared with the NBCIR. Known bortezomib related grade 3 toxicities are higher with BCIR recommending appropriate PN monitoring and HZV prophylaxis. The pooled estimates of response and survival strongly favor inclusion of bortezomib in the induction regimens.
Kaufman:Millenium, Onyx, Novartis, Keryx: Consultancy; Merck, Celgene: Research Funding. Gleason:Celgene, Merck, Millenium: Consultancy. Flowers:Genentech/Roche (unpaid): Consultancy; Celgene: Consultancy; Millennium/Takeda: Research Funding; Wyeth: Research Funding; Novartis: Research Funding. Lonial:Millennium: Consultancy; Novartis: Consultancy; Celgene: Consultancy; BMS: Consultancy; Onyx: Consultancy; Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal