Abstract
A prognostic scoring system for patients enrolled in phase I trials was developed by the Royal Marsden Hospital (RMH) (Arkenau H-T et al., 2008). The risk score consisted of a score of 0–3 based on the presence of three variables - elevated LDH, low albumin, and > 2 metastatic sites. Median survival for patients with score 0–1 vs. 2–3 was 33 weeks vs. 15 weeks. The RMH prognostic system was validated in a large group of patients at the MD Anderson Cancer Center Phase I Clinic (Stephen et al., 2011) and three additional independent negative prognostic variables were identified (ECOG PS > 1; > 3 prior lines and GI tumor site). Median survival in patients with score of 0 vs. 6 was 27 months vs. 9 weeks. These scoring systems are intended for patients with solid tumor malignancies, and no equivalent scoring system exists for hematologic malignancies. We propose a disease-specific prognostic scoring system for MM patients enrolled in phase I clinical trials.
In total, 21 phase I trials for relapsed/refractory MM from February 1, 2005 through August 2, 2011 were reviewed. Cutoff date for censoring was 08/02/2011. Autologous stem cell transplant was deemed as a line of therapy. In addition to the significant prognostic factors detailed, factors such as prior autologous stem cell transplant, prior radiation therapy, prior history of venous thromboembolism (VTE), monoclonal protein (IgA), BMI > 35 kg/m2, labs at enrollment (Hb<8 gm/dl, Plts<75K, corrected Calcium >10.2, ANC <2500, Creatinine>2, LDH>ULN) were considered in the model. Adverse cytogenetic abnormalities were defined as t (4;14), t (14;16) or del 17p diagnosed by FISH or presence of t (4;14), t(14;16), del 17p or complex karyotype diagnosed by conventional karyotyping.
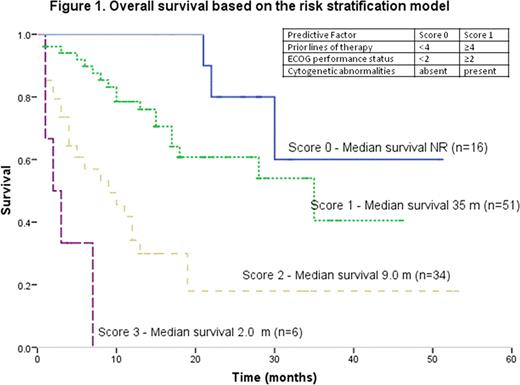
151 consecutive relapsed/refractory MM patients that were enrolled in at least one trial in the Phase I clinic were identified. The median patient age at the time of diagnosis and at the time of phase I trial enrollment was 57 years (range, 28–75) and 61 years (range, 34–80), respectively. Median time from diagnosis to enrollment in a phase I trial is 50 months (range, 3–154). The median number of cycles received on phase I trialswas 3 (range, 0–30) and the prior lines of therapy was 4 (range, 0–13). The median overall survival was 18 months (95% confidence interval, 14.18–21.82). 57 (47%) patients had high risk cytogenetics; 37 (26%) had Stage III ISS; 12 (9%) had ECOG PS ≥ 2 and 113 (75%) received ≥4 lines of therapy. In the univariate analysis, the factors that influenced the OS were lines of therapy (<4 vs. ≥4: 49 months vs. 13 months, p=0.001); ECOG performance status (PS) (<2 vs. ≥2: 20 months vs. 3.0 months, p=0.000), ISS (stage I vs. II&III: 40.21 months vs. 27.01 months, p=0.001; stage I&II vs. III: 35.23 months vs. 17.53 months, p=0.009); and cytogenetic abnormalities (standard risk vs. high risk: 34.07 months vs. 20.73 months; p=0.000). Using a multivariate Cox model, independent factors that predicted shorter survival were ≥ 4 prior lines of therapy (HR 3.44, 95% CI 1.44–8.21; p=0.005); ECOG PS ≥2 (HR 3.11, 95% CI 1.25–7.73; p=0.015) and presence of cytogenetic abnormalities (HR 2.43, 95% CI 1.23–4.80; p<0.011). We constructed a model to risk stratify patients at the time of enrollment using independent factors (with similar HR) influencing survival; with 1 point each allocated for ≥ 4 prior lines of therapy, ECOG PS ≥2 or presence of cytogenetic abnormalities. Median OS of 2 months (95% CI 0–4.4 months) was observed in patients with the highest cumulative score (3) while the median OS has not been reached in patients with the lowest cumulative score (0) as depicted in Figure 1.
We demonstrate the first predictive model of outcomes for relapsed/refractory MM patients in phase I studies incorporating routinely used clinical measures. This information can be used to accurately inform patients of potential outcomes when enrolling to phase I trials. A larger, prospective trial is needed to validate our model.
Kaufman:Millenium: Consultancy; Onyx Pharmaceuticals: Consultancy; Novartis: Consultancy; Keryx: Consultancy; Merck: Research Funding; Celgene: Research Funding. Flowers:Genentech/Roche (unpaid): Consultancy; Celgene: Consultancy; Millennium/Takeda: Research Funding; Wyeth: Research Funding; Novartis: Research Funding. Lonial:Millennium: Consultancy; Novartis: Consultancy; Celgene: Consultancy; BMS: Consultancy; Onyx: Consultancy; Merck: Consultancy.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal