Abstract
Myelofibrosis (MF) is a myeloproliferative neoplasm associated with splenomegaly, debilitating symptoms, cytopenias and progressive bone marrow fibrosis. Survival in MF is poor, and effective therapy is lacking. Ruxolitinib (INCB18424) is a JAK1 and JAK2 inhibitor with established clinical benefit in patients (pts) with MF (Verstovsek S. J Clin Oncol 29: [suppl; abstr 6500], 2011) by reducing spleen size and improving MF symptoms & quality of life.
Aim was to identify potential correlates of overall survival (OS) of MF pts receiving ruxolitinib. This study was based on a subset analysis of an open-label single-arm phase I/II trial (INCB18424–251; NCT00509899).
158 adult pts with primary or secondary MF were enrolled in the parent trial; most received ruxolitinib at doses of 10–25 mg PO twice daily. This updated analysis focuses on 107 pts enrolled at MDACC: 63 were high, 34 intermediate (int)-2 and 10 int-1 risk, according to the International Prognosis Scoring System (IPSS), and assesses their survival and correlates thereof. For log-rank survival analysis, events were censored at the later of last dose, last visit, or last follow-up date.
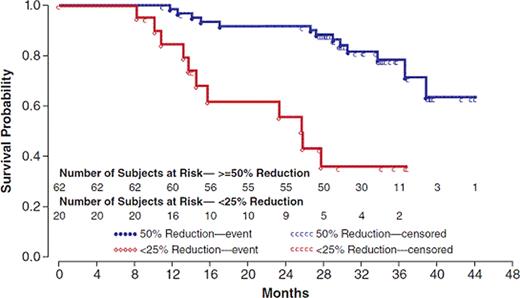
Efficacy and safety findings of the parent trial have been published (Verstovsek S. N Engl J Med 363:1117, 2010): ruxolitinib treatment led to a rapid and sustained reduction in splenomegaly and improvements in MF symptoms; anemia and thrombocytopenia were the most common adverse events. After a median follow-up of 32 months, 58 of 107 pts (54%) were still receiving therapy. The corresponding overall survival (OS) was 69% (33 pts died, none due to therapy-related reasons: 14 while on therapy/within 30 days (d) of discontinuation (dc), and 19 off-study). Accounting for deaths occurring on the study, the 2-yr actuarial survival of int-2 and high-risk pts was 92% and 88%, respectively. However, the 2-yr survival of 13 int-2 and 21 high-risk pts who had discontinued therapy and were subsequently followed was 32% and 21%, respectively. MF transformed to acute leukemia in 9 pts: 5 while on therapy/within 30 d of dc, and 4 off-study; the transformation rate was 0.036/pt years. Pts with normal baseline cytogenetics did not have better survival than those with aberrations (Hazard ratio [HR]=1.52; p=0.24). However, pts with a baseline bone marrow fibrosis score of 2 had greater survival than those with a score of 3 (HR=2.21; p=0.031). Other evaluable baseline pt characteristics (gender, age, anemia, WBC and splenomegaly, did not affect survival. Surprisingly, high-risk pts (per either IPSS or dynamic IPSS [DIPSS]) did not have significantly worse survival than int-2 pts. Importantly, reduction in palpable spleen length while on ruxolitinib was noted to be the most robust predictor for survival: pts who had a ≥50% reduction in spleen size (n=62) had significantly prolonged survival vs. those with a <25% reduction (n=20) (Fig. 1; HR=4.94; p<0.0001).
Kaplan Meier Plot of Survival in Patients with ≥50% and <25% Reduction in Palpable Spleen Length During Ruxolitinib Therapy
Kaplan Meier Plot of Survival in Patients with ≥50% and <25% Reduction in Palpable Spleen Length During Ruxolitinib Therapy
Most MDACC pts with advanced MF in the phase I/II ruxolitinib study are still receiving therapy, demonstrating an OS of 69% after a median of 32 months. The 2-yr survival of pts who remained on therapy was 3–4-fold greater than those who discontinued therapy. Among baseline pt characteristics, only a lower bone marrow fibrosis score correlated with better survival. Conversely, achievement of ≥50% reduction in spleen size while on ruxolitinib resulted in greater survival (vs. <25% reduction). Our data suggest that the most important factors that influence survival of MF pts receiving ruxolitinib are continuous active therapy and a degree of the spleen response, not pt pretherapy characteristics.
Verstovsek:Incyte Corporation: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal