Abstract
Abstract 3770
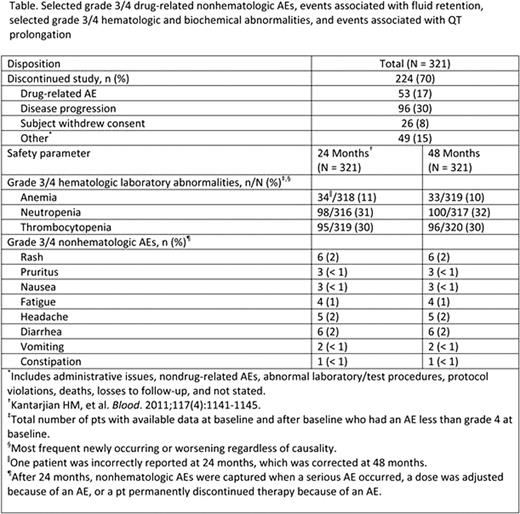
Nilotinib is a selective and potent BCR-ABL TKI approved for the treatment of pts with newly diagnosed Ph+ CML-CP, and for pts with CML-CP or CML-AP resistant to or intolerant of imatinib. Here, we present the 48-mo follow-up data from the 2101 trial for pts with imatinib resistance or intolerance. Methods: Pts were treated with nilotinib 400 mg twice daily (BID). Key endpoints included PFS (defined as progression to AP/BC or discontinuation due to disease progression as assessed by investigator or death from any cause) and OS (includes deaths during treatment or follow-up after discontinuation). Results: 321 pts were enrolled (70% imatinib resistant; 30% imatinib intolerant with resistance). At baseline (BL), 36% of pts were in CHR. At the time of data cutoff, 224/321 pts (70%) discontinued nilotinib therapy (Table), and 31% of all pts had at least 48 mo of treatment. The median nilotinib dose intensity was 789 mg/day (range, 151–1110) and 62% of pts received ≥ 400 mg BID nilotinib as their last dose available. Pts with BL CHR had a significantly higher PFS rate at 48 mo vs pts without BL CHR (71% vs 49%, respectively; P =.001). Only 11 (3%) pts progressed to advanced disease (AP/BC) during study. Estimated 48-mo OS rate was 78% (95% CI 74%-83%). Among resistant pts, those without BL mutations (n = 92) had a significantly higher OS rate at 48 mo vs pts with sensitive mutations at BL (n = 78) (84% vs 74%, respectively, P =.029); however, there was no significant difference in OS among pts with sensitive and insensitive mutations (Y253H, E255K/V or F359C/V, n = 27) at BL (74% vs 71%, respectively, P =.804). No new safety signals were observed, and few additional AEs were reported since 24 mo follow-up (Table). Biochemical lab abnormalities were generally mild, transient, and easily managed; grade 3/4 lipase elevation (19%), hypophosphatemia (18%), and hyperglycemia (13%) were most common. Reports of any-grade pleural effusions remained low (1%), and no new cases were reported with longer follow-up. No new cases of QTcF >500 ms and 3 new cases of QTcF increases > 60 ms from BL were reported. Nine pts died during treatment or within 28 days of discontinuation: 8 deaths were previously reported and occurred in the first 24 mo of follow-up; 1 additional death due to lung neoplasm occurred between 24 and 48 mo (35 mo). Conclusions: With longer follow up, nilotinib continues to be effective and well tolerated in pts with Ph+ CML-CP resistant to or intolerant of imatinib therapy. Nilotinib prevented progression to AP/BC in the majority of pts on treatment and was associated with high OS rates. No cumulative toxicity was observed. Data demonstrating the higher rate of PFS in pts who entered the study with a BL CHR suggest that switching pts to nilotinib prior to hematologic failure on imatinib, and according to current treatment guidelines, may maximize the efficacy of nilotinib therapy.
le Coutre:Novartis: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria. Giles:Novartis: Consultancy, Honoraria, Research Funding. Pinilla-Ibarz:Novartis: Research Funding, Speakers Bureau. Larson:Novartis: Consultancy, Honoraria, Research Funding. Gattermann:Novartis: Honoraria, Research Funding. Ottmann:Novartis: Consultancy; BMS: Consultancy, Research Funding. Hochhaus:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Radich:BMS: Consultancy; Novartis: Consultancy, Research Funding. Saglio:Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Pfizer: Consultancy. Hughes:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees. Martinelli:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy. Kim:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Branford:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Ariad: Research Funding. Müller:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Shou:Novartis: Employment. Novick:Novartis: Employment, Equity Ownership. Fan:Novartis: Employment. Cortes:Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ariad: Consultancy, Research Funding. Baccarani:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau. Kantarjian:Novartis: Consultancy, Research Funding; BMS: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal