Abstract
Abstract 368
Factor XII (FXII) participates in inflammation. FXII deficient patients have reduced leukocyte migration into skin windows. Recently, we have determined that FXII signals through uPAR, β1 integrin and the EGFR to stimulate ERK1/2 and Akt phosphorylation (Blood 115:5111, 2010). The downstream consequences of this pathway are FXII-induced cell proliferation and post-natal angiogenesis. Present studies examined if this pathway also influences leukocyte response to injury.
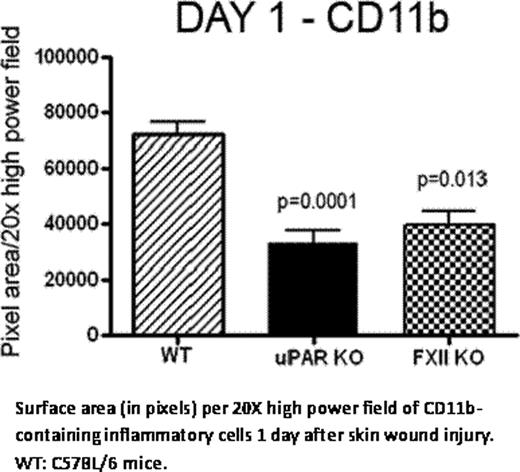
Exon 3- to 8-deleted FXII knockout mice (FXII KO), generously provided by Dr. Frank Castellino, have impaired inflammatory response to injury. FXII KO were wounded by creating full thickness (5 mm) punch biopsies on their backs. The total surface area of inflammatory cells recruited at the injury site/20 × high power field (HPF) was determined and analyzed by Image J (NIH). We observed that FXII KO on Day 1 exhibit significantly decreased recruitment of CD11b-labeled inflammatory cells to injury sites compared with wild type mice (WT) [mean ± SEM, FXII KO: 40220 ± 3732 vs. WT: 59740 ± 6318 total surface area of CD11b positive cells (pixels)/HPF, p=0.0136]. Similar results are observed when uPAR KO were compared to WT mice [39380 ± 5234 vs. 73310 ± 4688, p=0.0001], suggesting that leukocyte migration is mediated through uPAR. On Day 1 of thioglycolate (TG)-induced peritonitis, FXII KO have a mean of 12.62 × 105/mL peritoneal exudate cells (PEC) vs. 25.94 × 105/mL (p=0.0072) observed in WT. Additionally on Day 7, FXII KO have 10.63 × 105/mL PEC vs. WT 25.38 × 105/mL (p< 0.0001). Likewise on Day 7, uPAR KO have 16.34 × 105/mL peritoneal leukocytes recruited vs. WT 25.38 × 105/mL (p=0.0124). These combined studies indicate that FXII influences the degree of leukocyte inflammation and the inflammatory response is partially mediated through uPAR. Studies next determined if leukocyte recruitment promotes wound healing. On Days 3–5 the rate of wound closure of back punch biopsies is faster in FXII KO than WT (p < 0.0327 by one way ANOVA). Again, we observed that FXII KO on Days 2 and 5 exhibit significantly less CD11b-labeled inflammatory cells to the injury site compared to WT (Day 2: p=0.0004, Day 5: p= 0.0075). Leukocyte subpopulation analysis reveals decreased neutrophil migration (Gr-1 positive cells) into the wound area of FXII KO on Day 2 [FXII KO 5669 ± 1844 vs. WT 40490 ± 8564 total surface area of Gr-1 positive cells (pixels)/ HPF, p=0.0018] and Day 5 [FXII KO 6216 ± 3829 vs. WT 34890 ± 7629 total surface area of Gr-1 positive cells (pixels)/HPF, p=0.0176]. Macrophage recruitment into the wound area, as determined by F4-80 antigenicity, increases over time, but remains significantly reduced in FXII KO mice on Day 2 [FXII KO 3111 ± 1115 vs. WT 19140 ± 5767 total surface area of F4-80 positive cells (pixels)/HPF, p=0.0348] and Day 5 [FXII KO 9377 ± 4772 vs. WT 28340 ± 4411 total surface area of F4-80 positive cells (pixels)/HPF, p=0.0171]. These studies indicate that, in FXII deficiency, there is less neutrophil and macrophage-mediated inflammation and this observation correlates with faster wound healing. Finally studies determined if the reduced inflammation seen in FXII KO is the result of host factors or bone marrow-derived cells. Adoptive bone marrow transplant (BMT) experiments were performed where WT or FXII KO bone marrow was transplanted into FXII KO hosts. Eight weeks following the BMT, mice were subjected to TG-induced peritonitis. KO/KO recipients, have significantly decreased number of PEC on day 1 (17.3 × 105/mL ± 3.894 × 105/mL cells) and day 7 (18.6 × 107/mL ± 4.0 × 107/mL cells) when compared to WT/KO recipients [Day 1: 68.25 × 105/mL ± 13.83 × 105/mL cells (p=0.041), Day 7: 190.5 × 107/mL ± 51.80 × 107/mL cells]. These data indicate that FXII interacts with neutrophils and macrophages to promote the inflammatory response; its absence causes decreased inflammatory cell recruitment.
Our data indicate that FXII deficiency disrupts the leukocyte response to injury by reducing inflammatory cell recruitment in two murine models. Paradoxically, reduced leukocyte infiltration into skin wounds promotes healing. These investigations indicate a novel role for FXII in inflammation and wound healing and indicate a unique potential target for inflammation therapeutics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal