Abstract
Abstract 3645
The bulk of the tumour infiltrate in classical Hodgkin lymphoma (CHL) is composed of immune cells, predominantly CD4+ T cells, with the malignant Hodgkin Reed Sternberg cell (HRS) representing <1% of cells. The lymphoid microenvironment has been described as anergic and hypoproliferative with suppressive properties (Marshall et al. Blood 2004 103:1755–62) but the functional significance of this is unclear. This study set out to examine the proliferative capacity and phenotype of T cells derived from CHL-diagnostic lymph node tissue taken at diagnosis.
Frozen single cell suspensions (SCS) from 6 patients were selected from the tissue bank of our Institute. T cell growth-augmenting and/or Th2 polarising cytokines were added in various combinations (IL2, IL4 only, IL2+4 or no added cytokine) to SCS-derived cells in 96 well plates at 0.3 × 106 cells per well in 200mcl of optimized lymphocyte culture media. No CD4+ enrichment step was carried out: all recovered cells were plated at baseline to maintain potential interactions between CD4+ cells and other cells, and no mitogen or T-cell receptor-stimulating or costimulating agents were added at any point. As controls, SCS derived from normal tonsil, and ÔreactiveÕ lymph nodes (n=4) (confirmed by histological report at the time of diagnosis) were also plated. Plates were examined daily for cell/colony morphology to estimate growth and split with fresh media and cytokines once every 7 days, with estimated proliferation (by haemocytometry) plotted. Cultures were assessed at baseline, 10 days, 28 days, 50 days and 100 days.
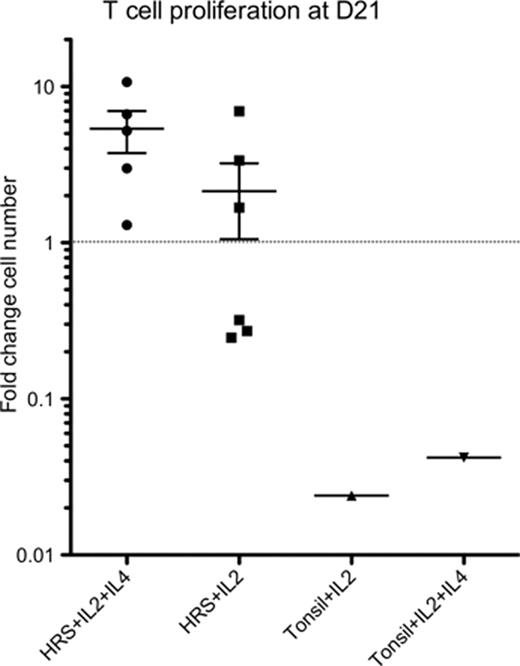
Proliferation, based on formation of discrete colonies and blastoid cell morphology, occurred in the majority of wells by day 7 in all CHL-derived cultures, and in a minority of wells, and to a lesser extent in all control cultures. CHL-derived T cells from one patient continue to expand after 200 days, doubling every 3–5 days, while the other 5 continue after 50–100 days. In contrast, no tonsil or reactive node-derived T cells survived beyond 50 days and none showed a net expansion in cell numbers. Growth was superior in the IL2+4 and IL2-only conditions, with no growth in the media-only or IL4-only conditions. The most favorable condition was with the addition of IL2+4. By day 21 a net increase in CHL-derived T cells was apparent, but not in any control T cell populations (Figure). At baseline, composition of the CHL-derived cells revealed a majority of CD3+ cells as expected, of which 60–80% were CD4+ and the remainder CD8+. By D21 the CD4+ component had outgrown all other cells in the CHL-derived cultures, being CD3+CD4+CD45RO+ consistent with antigen-experienced T helper cells, while all tonsil and reactive node-derived cells were CD8+CDRO+. Markers of central memory (CCR7 & CD62-L), Th2 (CCR4 and IL4), Treg (FOXP3 and CD25) and anergy (CD57) were absent after expansion, while markers of activation were upregulated (CD28, CD27, CD69, CD40L, CD30 & CD95). This phenotype persisted in the ongoing T cell lines.
The CD4+ compartment of the CHL microenvironment contains a primed subset of cells capable of massive expansion without further mitogenic stimulation and of generating cytokine-dependent continuous cell lines with an antigen-experienced, activated phenotype. This challenges the assumption of T cell anergy and hypoproliferation in the tissue microenvironment of CHL. We are currently assessing the function and anti-tumor specific or tumor supportive nature of these T cells.
Gribben:Roche: Honoraria; Celgene: Honoraria; GSK: Honoraria; Mundipharma: Honoraria; Gilead: Honoraria; Pharmacyclics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal