Abstract
Abstract 3636
Radioimmunotherapy (RIT) studies for the treatment of acute myeloid leukemia (AML) have mostly employed antibodies (Ab) labeled with 131I or 90Y, but both radionuclides have limitations. The high-energy gamma component of 131I requires that patients be treated in isolation to minimize radiation exposure risk to staff and family, warranting consideration of alternative radionuclides. In contrast, 90Y is a pure beta-emitter used in RIT, thereby not requiring extensive radiation isolation. It also has the potential of delivering improved therapeutic ratios of absorbed doses of radiation because of its higher beta energy (2.3 Median Energy [MeV] for 90Y compared to 0.66 MeV for 131I) and shorter half-life (2.7 days for 90Y compared to 8 days for 131I). Because 90Y has few gamma emissions, a surrogate radioisotope is required for nuclear imaging, necessary for dosimetry calculations. In view of these limitations, and the anti-tumor effects of 131I-anti-CD45 Ab demonstrated by our group, we studied 177Lu, a beta-emitting radio-metal with a half-life (6.7 days) and energetics (0.5 MeV) similar to 131I. However, 177Lu has lower energy gamma emissions during its decay that is theoretically capable of reducing whole body exposure to gamma radiation while facilitating imaging. To optimize the therapeutic efficacy and minimize toxicities of RIT we compared the relative merits of 90Y and 177Lu in biodistribution, dosimetry, and therapy experiments using a mouse model of syngeneic, disseminated AML.
For biodistribution studies, we inoculated B6SJLF1/J mice with 105 leukemic PBMC via tail vein injection and two days later mice received 90Y- or 177Lu-DOTA-30F11 (anti-murine CD45 Ab). Mice were euthanized at serial time-points, their organs harvested and then analyzed in a gamma counter to calculate the percent of the injected dose of radiolabeled Ab per gram of each organ (%ID/g). In therapy studies, 10 mice per group were inoculated with leukemic PBMC, and two days later treated with either 100 mCi or 300 mCi of90Y- or 177Lu-DOTA-30F11.
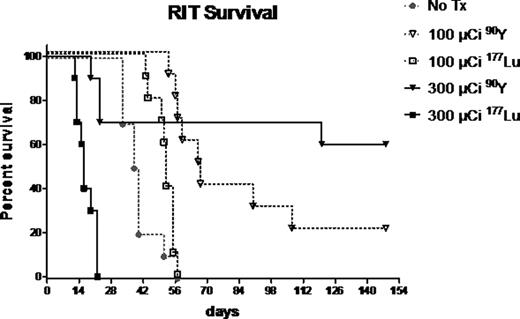
Biodistribution studies demonstrated excellent localization of both radioimmunoconjugates to sites of highest leukemic burden (bone marrow [BM] and spleen), while minimizing uptake in non-hematopoietic organs. After 24 hours, 67.5 ± 19.0% and 187 ± 20.2% ID/g of 90Y-DOTA-30F11 and 9.8 ± 2.7% and 141 ± 18.3% ID/g of 177Lu-DOTA-30F11 were delivered to BM and spleen, respectively. Non-hematopoietic organs such as kidneys had ≤5.2 ± 0.8% ID/g for 90Y and ≤2.7 ± 0.3% ID/g for 177Lu. This approach yielded BM-to-normal organ ratios of %ID/g as high as 36:1 (90Y) and 39:1 (177Lu) for muscle. Ratios were higher when comparing spleen to normal organs: 375:1 (90Y) and 409:1 (177Lu) for muscle. Ratios for dose limiting organs such as liver and lung were also favorable, with spleen:lung = 46.9:1 and BM:lung = 4.6:1 for 90Y, and spleen:lung = 47.8:1 and BM:lung =4.8:1 for 177Lu. In therapy studies, all untreated AML mice (Figure - No Tx group) died of disease between 33 and 56 days (median 39 days). Sixty percent of mice that received 300 mCi and twenty percent of mice that received 100 mCi of 90Y-DOTA-30F11 are alive over 140 days after therapy (median survival not reached and 67 days, respectively). Conversely, all mice that received 300 mCi of 177Lu-DOTA-30F11 died of toxicity and survived a median of only 16 days while all mice treated with 100 mCi of 177Lu-DOTA-30F11 died due to progressive AML (median survival 52 days).
These data suggest that 90Y-anti-CD45 RIT may be more effective and better tolerated than 177Lu-anti-CD45 RIT. These data also suggest that the toxicities seen in this model using 177Lu may be attributed to its longer half-life compared to 90Y, while the inferior efficacy detected may be a consequence of the lower overall dose delivered to AML cells in vivo. The superiority of 90Y compared to 177Lu for use in RIT is being confirmed using a human AML xenograft system. 90Y and 177Lu are also being compared using a pretargeted RIT approach in both murine leukemia models.
Gopal:GSK: Research Funding; Spectrum: Research Funding; Seattle Genetics: Consultancy, Research Funding; Merck: Research Funding; Piramal: Research Funding; Cephalon: Research Funding; Millenium: Speakers Bureau; Abbott: Research Funding; Pfizer: Research Funding; SBio: Research Funding; Bio Marin: Research Funding; Biogen-Idec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal