Abstract
Thrombopoietin (TPO) is the major regulator of platelet production. In prior clinical studies, thrombopoietin levels have been shown to vary inversely with circulating platelet mass and with the rate of platelet production. Thus, TPO levels may help distinguish between the various disorders of thrombocytopenia. In addition, the introduction of TPO agonists has created an interest in predicting the response of patients to these agents. Determining TPO levels may help predict such treatment responses.
Sera from 121 patients with a history of abnormal platelet counts were tested using a novel, commercially available ELISA assay that measures TPO levels. The TPO assay detected TPO levels as low as 7 pg/mL and was linear for levels up to 2000 pg/mL. The coefficient of variation ranged from 27% near the lower limit of detection to 9% at a TPO concentration of 669 pg/mL. The reference range for TPO was established in serum samples from 118 apparently healthy individuals (58 males and 60 females) and was 7–99 pg/mL. The Wilcoxon test was used to compare continuous variables and the Fisher's exact test was used to compare categorical variables.
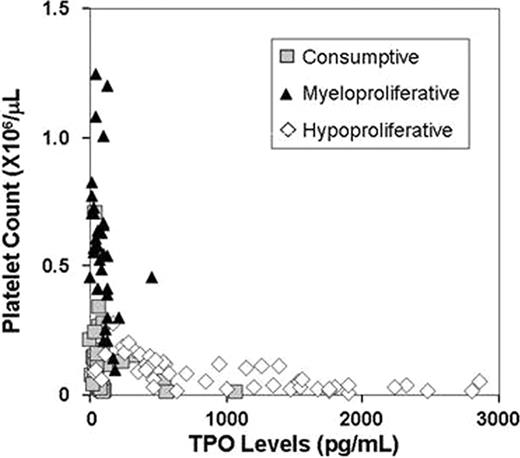
The patient population included 40 patients with a consumptive thrombocytopenia (38 with primary or secondary immune thrombocytopenic purpura (ITP), 2 with thrombotic thrombocytopenic purpura), 34 patients with myeloproliferative disorders (23 with essential thrombocytosis, 9 with polycythemia vera, 2 with an ill-defined myeloproliferative disorder), and 47 patients with hypoproliferative thrombocytopenia (29 with chemotherapy-related thrombocytopenia, 19 with primary or secondary bone marrow failure syndromes). Among the 38 patients with ITP, 11 were taking TPO agonists (9 on romiplostim, 2 on eltrombopag), 19 were taking immunomodulatory agents (16 on steroids alone or in combination with other therapies, 2 on azathioprine, 1 on danazol), and 12 were off ITP-specific therapy when the TPO level was measured. 9 out of 38 (24%) patients with ITP had undergone splenectomy and/or been previously treated with rituximab. The median serum TPO level in patients with consumptive thrombocytopenia was 64.5 pg/mL (interquartile range, 48.5–97.5 pg/mL) and the corresponding median platelet count was 68,000/μL (interquartile range, 27,000–144,500) (Figure). While patients with myeloproliferative disorders had similar TPO levels [median 87.0 pg/mL (38.0–125.5)], their platelet counts were significantly higher than those of patients with consumptive thrombocytopenia [median 549,500/mL (431,250–693,000] (P <0.0001). Contrastingly, comparable platelet counts [median 61,000/μL (31,000–118,000)] were observed among patients with hypoproliferative thrombocytopenia, but serum TPO levels were significantly higher than those of patients with consumptive thrombocytopenia [844 pg/mL (409.5–1551.5), P <0.0001].
Among 22 evaluable patients meeting diagnostic criteria for primary or secondary ITP who had taken a TPO agonist for at least 1 month, serum TPO levels appeared to predict responsiveness to the drug. A clinical response to a TPO agonist was defined as achieving a platelet count ≥50,000/μL after starting the drug and maintaining it at or above that count in ≥50% of subsequent complete blood counts from initiation until discontinuation of the drug, loss to follow-up, or 6 months had passed, whichever was longest, without the need for recurrent rescue therapy. Whereas 14 out of 16 (88%) ITP patients with a TPO level <99 pg/mL met our definition for a clinical response to treatment with a TPO agonist, only 1 out of 6 patients (17%) with a TPO level >99 pg/mL responded (P <0.005 for the difference in clinical response to TPO agents.)
TPO levels may have diagnostic utility in discriminating between patients with hypoproliferative and consumptive thrombocytopenia. High TPO levels among patients with ITP may predict a poor clinical response to treatment with TPO agonists. Further studies are required to confirm these data.
Zhukov:Quest Diagnostics: Employment. Sahud:Quest Diagnostics: Employment. Kuter:Quest Diagnostics: Consultancy, Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal