Abstract
Abstract 3258
At sites of vascular injury, platelets are exposed to multiple agonists that lead to overall activation and platelet plug formation. Signaling pathways induced by these agonists are known to interact with each other. For example, collagen binding to the platelet collagen receptors, α2β1 integrin, CD36, and glycoprotein VI, induces inside-out signaling that ultimately leads to the activation of the glycoprotein IIb/IIIa receptor for fibrinogen on the platelet surface (Nakamura et al, JCB, 1999).
An assay capable of tracking the biological effects of multiple different agonists simultaneously within a single platelet would enhance our understanding of how platelets integrate these different signals at the single-platelet level. This assay would have advantages over current clinical assays in that platelet function tests such as platelet aggregometry or the PFA-100 assess only collective behavior of platelet populations without single platelet resolution. Flow cytometry achieves single platelet resolution but cannot monitor the dynamic changes induced by agonists over time. Platelet adhesion assays have the capability to track individual platelets over time via microscopy, but currently cannot simultaneously monitor the different effects of multiple agonists and their potential interactions.
To that end, we developed a modified platelet adhesion assay by using microcontact printing to “stamp” distinct micro-to-nanoscale patterns of different platelet agonists and ligands on glass coverslips. Different parts of each platelet are then exposed to different agonists/ligands with our assay and how the different downstream biological signals interact, synergize, and potentially compete can be monitored overall time at the single platelet level via epifluorescence microscopy.
Using microfabrication techniques, patterned polydimethylsiloxane (PDMS) stamps were” inked” with two different known platelet agonists/ligands: Collagen type 1 conjugated with FITC (green) and fibrinogen conjugated to Alexa Fluor 594 (red). Using those stamps, protein micropatterns were then “microstamped” and transferred onto the glass surface, creating spatially distinct microprints with two different proteins (Fig. 1). The protein surface was then blocked with 1% BSA.
Platelets were isolated and dyed with a fluorescent membrane marker. Platelets were suspended in Tyrode's buffer (20 million platelets per mL) and incubated with the double micro-patterned surface. After incubation, the surface was washed and imaged with epifluorescence microscopy using a 40x objective.
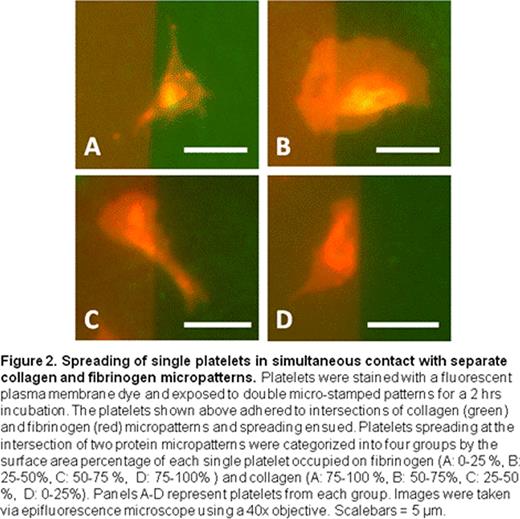
For these initial proof-of-concept experiments, we observed that when individual platelets are simultaneously exposed to separate areas of fibrinogen as well as collagen, they exhibit a strong “preference” for the fibrinogen over collagen (Fig. 2). Indeed, 79.1% of platelets were observed to almost completely (>75% of the surface area) migrate to the fibrinogen micropattern from the collagen micropattern (Fig. 3). Few platelets were spread equally between the two protein micropatterns, which suggest that platelets eventually “choose” which agonist to settle before it get fully spread and immobilized. Interestingly, platelets with more than 75 % surface area on collagen (8.96 % of total platelets measured) tended to be smaller in size, have filopodia, and have intense granule staining compared to platelets on fibrinogen, which were more fully spread with broad lamellipodia. These data suggest that the platelets that migrated to the fibrinogen micropattern compared to those that preferred collagen may be physiologically distinct.
Conclusions and Ongoing Efforts: These data establish the viability of our system to investigate the integrative effects of different agonists at the single platelet level. This assay will enable the further understanding of how different agonist-induced signaling pathways interact. Ongoing experiments will include other agonists/ligands such as von Willebrand factor and thrombin. Although the current data assesses morphologic changes of platelets, we are focusing our efforts on using high resolution fluorescence microscopy to measure and monitor the spatial dynamics of calcium signaling, protein phosphorylation of relevant signaling pathways, and cytoskeletal rearrangement when different parts of single platelets are exposed to different agonists.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal