Abstract
Abstract 3072
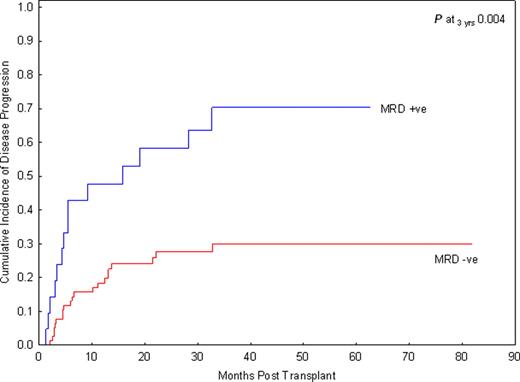
Allogeneic hematopoietic stem cell transplantation (SCT) is the only effective treatment for high-risk or relapsed acute lymphoblastic leukemia (ALL). However, SCT carries high treatment-related mortality (TRM). There have been continuing efforts to refine criteria for patients who may benefit most from SCT. Minimal residual disease (MRD) at the end of induction or induction/consolidation therapy has emerged as the most significant risk factor for disease relapse in ALL patients, and has become an indicator for SCT or intensified chemotherapy. In addition, MRD prior to SCT is highly predictive for post-transplant relapse in the pediatric population. The significance of pre-SCT MRD in adult patients with ALL remains to be determined. Patients and methods: In this retrospective analysis, we examined the impact of MRD detected within one month prior to SCT on risk of disease progression in adult patients with ALL who were in complete remission, defined by less than 5% blasts in bone marrow by morphologic assessment and normal blood counts at time of SCT. MRD has been uniformly assessed in patients using multiparameter flow cytometry since 2004. Bone marrow aspirates were stained using a comprehensive panel of >=15 markers and 200, 000 cells were analyzed. MRD was scored as positive based on a distinct cluster of >=20 cells, showing a significant difference in the level of expression (>=3-fold) of >=2 antigens, in comparison to the known phenotype of benign immature B-cell precursors. Disease progression (relapse) was defined as leukemic blast count >= 5%. Therefore, limiting our data set to transplants performed during February 2004 through May 2010 at MD Anderson Cancer Center, we identified 98 patients with median age 34 years (range 19–68) treated with a median of 2 lines of chemotherapy (range 1–6) who received a matched sibling (n=51), matched unrelated (n=36), haplo-identical family (n=3), or cord blood donor (n=8) transplant for ALL of B-(n=86) or T-lineage (n=12) in CR1 (n=52), CR2 (n=39) or CR3 (n=7) using a myeloablative non-TBI based (n=62) or TBI-based (n=36) transplant conditioning regimen. GVHD prophylaxis was tacrolimus-based for all patients. Among 88 patients with available cytogenetic data at diagnosis, 59% were considered high risk defined by the presence of the t(9;22), t(4;11), hypodiploid or complex cytogenetics, 2% were classified as good risk by the presence of hyperdiploidy, and the remaining 34% were intermediate risk. Results: Ninety-two patients were evaluable for response and all had sustained CR following transplant; 6 had early death. With a median follow-up of 33 months among survivors (range 5.8–80.7), patients transplanted without detectable MRD at time of SCT had a significantly lower cumulative incidence of relapse than those transplanted with MRD, 30% versus 70%, p=.004 (Figure 1). There was also a trend for better 3-year PFS in the MRD negative group, 38% versus 13%, p=0.1. Additional factors, including disease stage at time of SCT, cytogenetic profile at diagnosis, donor relation, and development of GVHD were evaluated in a univariate analysis for relapse rate, PFS, and overall survival. Only the presence of MRD remained a significant predictor for relapse. Conclusion: This study demonstrates that the presence of MRD prior to SCT in adult ALL patients is a significant predictor for relapse. Improvements in our current therapies are urgently needed to improve the outcomes for MRD positive patients. Close modal
Figure 1.
The cumulative incidence of disease progression in adult patients with ALL undergoing SCT in remission is stratified by MRD.
Figure 1.
The cumulative incidence of disease progression in adult patients with ALL undergoing SCT in remission is stratified by MRD.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal