Abstract
Abstract 2970
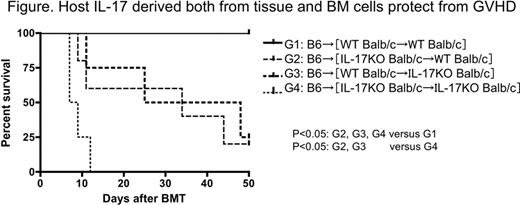
Th17 is a newly identified T cell lineage, which secretes the proinflammatory cytokine IL-17. Th17 have been shown to play a crucial role in some immune-mediated diseases. We have reported that host-derived IL-17 has a protective effect against acute graft-versus-host disease (GVHD). In contrast, donor-derived IL-17 exacerbates chronic GVHD in rodent models. Briefly, in acute GVHD model, lethally irradiated IL-17 knockout (KO) recipient mice receiving allogeneic BM with low dose splenocytes developed more severe acute gut GVHD compared to wild type (WT) host mice and finally half of them died (p<0.05). To demonstrate the mechanisms of protective role of host derived IL-17 against acute GVHD in further detail, first we determined in vivo gut epithelial permeability by using FITC-dextran. No significant difference was observed between WT mice and IL-17KO mice, even in GVHD induced mice. Next, to exclude the possibility that alloreactivity of host IL-17KO derived mature dendritic cells (DCs) could be much more than that of WT DCs, mixed leukocyte reaction (MLR) was performed using stimulators from WT or IL-17KO mature DCs and responders from WT allogeneic splenocytes. There was no significant difference between WT mature DCs and IL-17KO mature DCs in thymidine uptake and percentage of responder cells producing IFN-γ or TNF-α. However, when DCs were cultured from BM cells with GM-CSF, co-stimulatory molecules such as CD80 and CD86 were expressed much stronger and from earlier time point on IL-17KO DCs compared with WT DCs. This suggested that DCs from IL-17KO tend to be activated much easier, resulting in much severe GVHD. In addition, when recombinant IL-17 was added into MLR mixture (Stimulator: Balb/c IL-17KO mature DCs, Responder: B6 IL-17KO splenocytes), proliferation was significantly decreased, demonstrating that IL-17 has inhibitory effect on alloreaciton. Next, much higher number of infiltrated monocyte/macrophage cluster was observed in spleen and gut in IL-17KO host mice. Residual host-typed peritoneal macrophages in IL-17KO host mice were highly activated with the expression of TNF-α, while activation of donor-typed macrophages was much less with the production of anti-inflammatory cytokine IL-10. Activated monocytes/macrophages in IL-17KO recipients might migrate into inflamed tissue much easier, partially because lack of IL-17 dose not block some macrophage chemotactic factors such as IP-10, RANTES. Finally to explore which host IL-17 producing cells are necessary for protection from acute GVHD, that is, BM-derived IL-17 producing cells or tissue derived IL-17 producing cells such as Paneth cells in the gut, [IL-17KO→WT], [WT→IL-17KO], [WT→WT], and [IL-17KO→IL-17KO] chimeric mice (Balb/c background) were created by reconstituting lethally irradiated WT or IL-17 KO Balb/c (H-2d) with BM cells from WT or IL-17 KO Balb/c mice. Four months later, chimeras were re-irradiated with 8Gy TBI and injected with WT C57BL/6(H-2b) BM plus low dose splenocytes to induce acute GVHD. Although [WT→WT] chimeric recipients were all alive, all [IL-17KO→IL-17KO] chimeric mice died of severe GVHD. In both [WT→IL-17KO] and [IL-17KO→WT] chimeras, half of them died with less GVHD compared to [IL-17KO→IL-17KO] recipients as shown below. This result indicated that as well as tissue derived IL-17, host BM derived IL-17 is necessary for protection from acute GVHD. In conclusion, host-derived IL-17 has a protective effect against acute GVHD due to in part by regulating APCs (monocytes/macrophages/DCs) activation and migration into GVHD target tissues.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal