Abstract
Abstract 2949
The treatment of patients with newly diagnosed multiple myeloma (MM) with a 3 week cycle of lenalidomide (R), bortezomib (V), and dexamethasone (D) (R 25 mg days 1–14, V 1.3 mg/m2 day 1, 4, 8, 11, and D 20 mg day 1, 2, 4, 5, 8, 9, 11, 12) is associated with unprecedented response rates (Richardson et al. Blood 2010) (see Table 1). Partial response (PR) was 100% and 74% of patients achieved a very good partial response (VGPR) or better with RVD treatment. However, these responses come at the expense of an 80% rate of sensory neuropathy (27% grade 2 and 2% grade 3) and 32% with neuropathic pain (11% grade 2, 3% grade 3). As a result, only 59% of patients received all 3 agents and at least 1 dose modification of bortezomib was required in 44% of patients including 15% for neuropathic pain, 14% for sensory neuropathy, and 8% for fatigue Recent data confirm that a decrease in the dose intensity of bortezomib (for example with weekly dosing) is associated with significantly less toxicity, but importantly (at least in the context of combination chemotherapy) without a decrease in efficacy (Bringhen et al., Blood 2010). In this retrospective study, we examine the efficacy and toxicity of a 28 day RVD treatment schedule for patients with newly diagnosed MM.
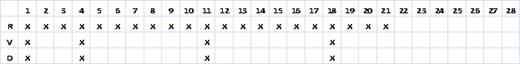
All patients with newly diagnosed, symptomatic MM who received front-line RVD on a 28 day schedule where R 25 mg was given days 1–21, V 1.3 mg/m2 was given on day 1, 4, 11, 18 and D 20 or 40 mg was given on day 1, 4, 11, 18 (see Table 2) were selected for this analysis. Patients were excluded if they had received any previous systemic anti-MM therapy (except prior corticosteroids for hypercalcemia or spinal cord compression). Patients who were empirically dose reduced for comorbidities were not excluded. All patients received prophylaxis for venous thromboembolism and VZV.
A total of 38 patients met the inclusion criteria for analysis. The median age at start of treatment was 59.5 years. The ISS distribution was Stage I 73% of patients, Stage II 16%, and Stage III 11%. Median treatment length was 4.5 cycles (range 3–12). After four cycles of treatment with VRD, the overall response rate was 97%, which included 63% VGPR or better (24/38) and 34% PR (13/38). 1 remaining patient had an MR (49% reduction in m spike). 32% of patients (n=12/38) went on to stem cell transplantation after four cycles of treatment while the rest were placed on maintenance therapy. With a median follow up of 9 months, no patients have had disease progression. Impressively, the rate of all grades of neuropathy at four cycles was 37% (n=14/38) with 21% (n=8/38) grade 1 sensory neuropathy, 11% (n=4/38) grade 2 neuropathy with pain and 5% grade 3 (n=2/38). There were no other hematologic or non-hematologic grade 3 or 4 toxicities.
Despite a decrease in the dose density of bortezomib and dexamethasone, the continued synergy of these agents with a full 21 day course of lenalidomide provides excellent efficacy with reduced rates of toxicity. The 28 day schedule of RVD is an efficacious, convenient, and well tolerated treatment regimen for patients with newly diagnosed symptomatic MM. The subcutaneous administration of bortezomib on this 28 day schedule may allow even further reductions in toxicity without sacrificing efficacy.
Chari:Millenium Takeda: speakers bureau (terminated May 2010); Celgene: lecturer. Jagannath:Celgene: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal