Abstract
Abstract 2845
Telomere length (TL) at diagnosis has been established as an independent outcome predictor in CLL (Rossi et al Leukemia 2009). However data on TL dynamics over time are scant and anedoctal. Aim of this study was to evaluate telomere dynamics in the natural history of CLL. This issue has been here addressed on a series of 88 CLL patients (pts).
25 pts were assessed for TL at diagnosis and at relapse and 63 pts had two determinations during the “watch and wait” (WW) phase. The series was fully characterized in terms of Binet stage, ALC, CD38, ZAP-70, IGHV mutational status (IGHV-MS), stereotyped receptors, cytogenetics and detailed clinical history. LDH, B2-microglobulin, p53 mutations and CD49d were available in more than 70% of pts. Treatment-free survival (TFS) analysis was performed exclusively in pts undergoing kinetic evaluation during the WW phase. This population had a median follow-up of 73 months and a median TFS of 130 months. TL was analyzed as previously described (Rossi et al Leukemia 2009; Ladetto et al Blood 2004). Median time between TL determinations was 44 months (range 12–231). Telomere loss was calculated in terms of both absolute loss (AL) and yearly loss (YL). Continuous variables were compared by the Mann-Whitney test, while TFS by the stratified Kaplan-Meyer method.
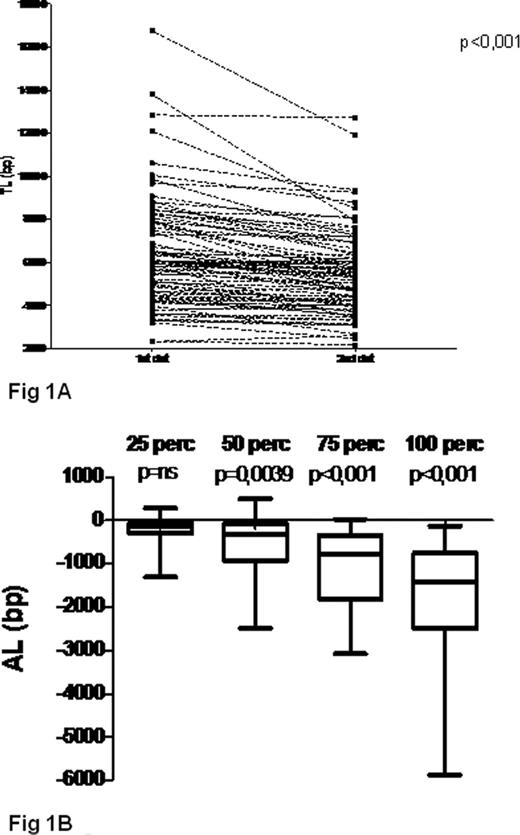
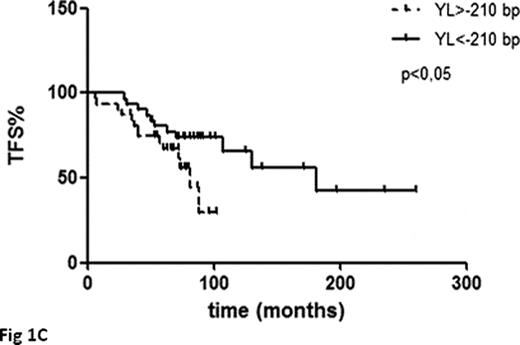
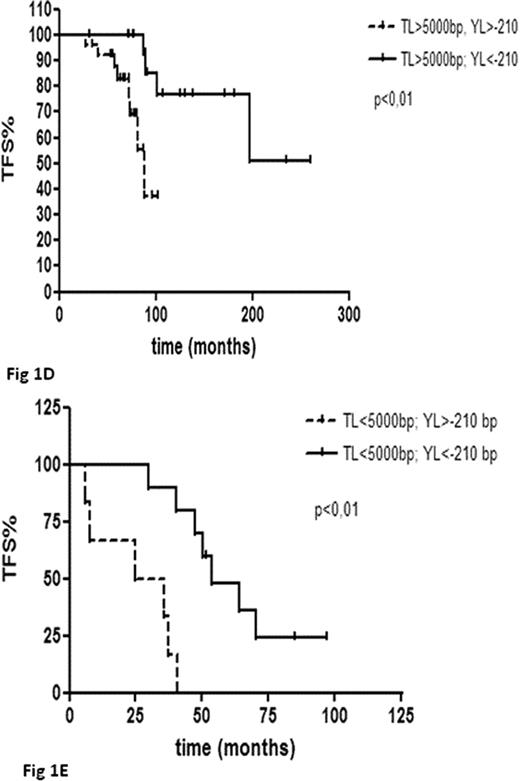
Telomeres were shorter at follow-up compared to baseline with a median loss of 651bp (range +493bp, −5874bp; p<0.001) (Fig 1A). AL and YL were greater in cases with higher baseline TL while those with short telomeres at diagnosis had only modest additional erosion (p=ns for pts in the 25th lowest percentile) (Fig 1B). Telomere loss over time was noticeable both in pts assessed at diagnosis and at relapse as well as in those assessed during the WW phase, but clearly inferior in the former subgroup (YL of −61bp, p<0.05 and −210bp, p<0.01, respectively), possibly due to the higher number of patients with short telomeres. AL and YL did not correlate with any available clinical or biological parameter, with the exception of a positive association with IGHV-MS (p<0.05). Pts with baseline TL shorter than the validated cut-off value of 5000bp (Rossi et al Leukemia 2009) were associated to an inferior TFS (median TFS 41 months vs 182 months; p<0.0001) as expected. Moreover also Binet status and IGVH-MS were predictive for TFS in this series. Surprisingly, also an YL above the median value (-210bp) appeared to be predictive for an inferior TFS (median TFS 82 months vs 182 months; p<0.05) (Fig 1C), despite being more common in pts with longer telomeres and VH-mutated IgH genes. Following stratification of pts according to baseline TL (< or > 5000bp), YL was predictive for TFS in both pts subgroups (Fig 1D i.e. baseline TL >5000bp; YL ≥ −210bp vs YL <-210bp: TFS 88 months vs not reached p<0.01. Figure 1E i.e. baseline TL <5000bp; YL ≥-210bp vs TL <210bp: median TFS 36 months vs 50 months, p<0.01).
The results of the first systematic analysis on TL dynamics in CLL indicate the following: i) progressive telomere erosion occurs as part of the natural history of CLL; ii) telomere loss is more pronounced when baseline TL is higher; iii) accelerated telomeric loss associates to an inferior TFS. The results described in the present analysis corroborate basic studies suggesting that telomere disruption represents a critical step associated to CLL progression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal