Abstract
Abstract 2798
MDS is a disease characterized by an increased level of bone marrow (BM) apoptosis, leading to the failure of normal haematopoiesis and accumulation of reactive oxygen species (ROS), resulting in DNA damage. NRF2 acts first as a sensor of “redox statusâ€□, then can activate genes implicated in the response to the oxidative stress. P53 activation, followed by increased levels of p53 protein, and subsequent apoptosis, is one of the responses to the oxidative stress, creating a circle. Iron overload is one of the mean causes of ROS accumulation in MDS apoptotic pathway and has been hypothesized the responsible of apoptosis in MDS. We studied the associations between ROS, p53 and iron overload in MDS.
Gene expression profiling was performed in of 62 newly diagnosed patients (pts) with MDS (N=52) and AML transformed from MDS (N=16) and (N= 8) normal donors for ROS-induced genes Nrf2, Keap1 and GSTiPi, and the tumor suppressor TP53. CD34+ cells were sorted from pts and normal donor bone marrow. RNA was isolated using standard protocols and cDNA was obtained by reverse transcriptase using the Invitrogen SuperScript_ One-Cycle cDNA Kit. Gene expressions were analyzed by qRT-PCR using Applied Biosystems Real-Time PCR 7900 and SYBR_ GreenERâ„¢ Reagent System from Invitrogen. Patient clinical and clinical-laboratory characteristics, including IPSS, blood counts, bone marrow features, serum ferritin were used for analysis. Patients were followed for time-to-event endpoints, including time to transformation to AML and survival, dated from initial sample collection
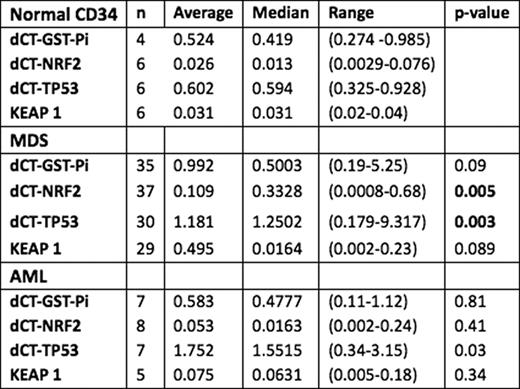
The median pt age was 68 yrs (range 32–91); 55% were male. The median % bone marrow (BM) blast count was 3.5% (0–19) for MDS and 16% for AML (0–16). Low-risk cytogenetics by metaphase karyotype was noted in 54%, intermediate-risk in 5%, and high-risk in 41%. IPSS score was high for 11%, Int-1 30%, Int-2 39%, and low for 20% pts The median ANC for MDS was 1448/mL (90–39245) and 1136 (90–39245) for AML; hemoglobin was 9.9 g/dL (6.4–14.6) and 9.45 g/dL (7–13.2) for MDS and AML; platelet was 57 k/_L (6–1040) and 57.5 k/_L (9–613) for MDS and AML, respectively. The median ferritin level was 867 ng/mL (24–14325) and 1136 for AML (159–3585); 39/52 MDS (69.7%) had ferritin levels higher than the normal range (p<.0001). The mean transcript levels for the genes are reported in the Table. The p value refers to the comparison of values with normal donors. The relative mean value of all the genes was higher than in normal CD34, although statistical significance was observed only for TP 53 (p.003) and NRF2 (p=0.005). In MDS, there was no significant correlation between serum ferritin levels and transcript levels of NRF2 (p=. 69), GSTiPi (p=.69), TP53 (p=.58) or KEAP1 (p=.66). In 62% 21/35 MDS pts BM blasts >10% correlated with higher Nrf2 levels (p=.03). The median follow-up time is 34 months (range 2–123); transformation to AML occurred in 16 pts and 22 patients overall (19 MDS and 3 AML) have died. The median survival time is 24 months for MDS and 19 months for AML. Patients with lower ferritin levels had a longer overall survival (50 mo vs 18) p=0.009. There was shorter survival (median 12 mo vs 20 mo p=0.007) or time to transformation (TTT) (median 8 mo vs 15 mo p=0.307) associated with higher ferritin levels (median 12 mo vs 20 mo p=0.007) and trend with higher NRF2 transcript levels.
CD34+ cells from BM of patients with MDS have higher transcript levels of Nrf2 and TP 53 than normal donor CD34+, increased NRF2 transcript to higher % BM blasts. There was no correlation between increased ferritin and over-expression of NRF2 and TP53. These data indicate that ROS activation and induction of apoptosis in the BM of MDS and AML are correlated with blasts number and characteristics independently of iron overload. Ferritin levels are higher in MDS and correlate with shorter survival and TTT.
Wierda:GlaxoSmithKline: Research Funding. Kantarjian:Eisai: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal