Abstract
Abstract 2677
Bendamustine (B) and rituximab (R) in combination have relevant clinical activity in mantle cell lymphoma (MCL), and a favorable toxicity profile. We recently demonstrated that B and cytarabine, a key drug in the treatment of younger patients with MCL, are strongly synergistic when added to MCL cell lines in-vitro. In the present study we combined cytarabine with B and R (R-BAC) in previously untreated patients with MCL aged >65, and in patients relapsed or refractory (R/R) to one previous line of immunochemotherapy.
This prospective safety/efficacy phase 2 study started with a dose-finding stage based on three stepwise dose-escalations of cytarabine. After the first 6 patients (3+3) were enrolled and concluded their whole treatment, the first dose level of cytarabine (800 mg/m2) was validated for the extension of the study. Therefore, all patients received 4 to 6 cycles of R 375 mg/m2 on day 1, B 70 mg/m2 on day 2 and 3, cytarabine 800 mg/m2 on Day 2, 3, and 4. Cycles were administered every 4 weeks, mostly on outpatient basis. The primary objectives were the safety of R-BAC, and assessment of overall and complete response rates (ORR and CRR).
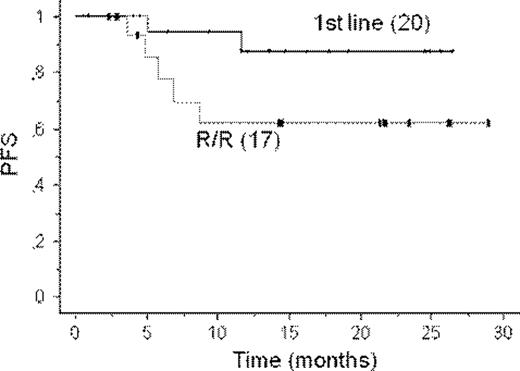
Thirty-seven patients have been enrolled, of whom 20 were previously untreated, and 34 were evaluable for response. Median age was 71 years (range 55–88), and 60% were males. Ann Arbor stage was III-IV in 92%, 44% had bulky disease, and MIPI was low/interm/high in 28/18/54% of patients, respectively. Cytologic subtype was blastoid in 17%, and mean ki-67 was 25% (range 5–80). All R/R patients had received rituximab containing regimens as first line. Six of them (35%) were refractory to induction therapy. Overall, R-BAC was very well tolerated. The dose limiting toxicity was hematological, with 60% and 90% of patients experiencing transient grade 3–4 neutropenia and/or thrombocytopenia, respectively (median duration 2 days, range 1–5). Grade 2 anemia was also frequent, with 60% of patients that were transiently treated with erythropoetin. Hematologic toxicity was significantly more frequent in R/R patients than in treatment naive. Three patients (8%) had febrile neutropenia with pneumonitis in one. Thirteen patients (35%) had grade 1–3 elevation of gamma-GT. No other significant toxicity was observed. Considering all patients, ORR was 88%, and CRR was 74% (CR 53%, unconfirmed CR 21%). CRR was 82% for previously untreated and 67% for R/R patients. According to the recently revised PET-including response criteria (Cheson BD et al, JCO 2007), CRR was 88% for previously untreated patients, and 73% for R/R. At a median follow-up from the start of therapy of 13 months (range 1–28) the 1-year progression-free survival was 76% ± 8% (87% ± 8% for previously untreated, and 62% ± 13% for R/R, Figure 1).
R-BAC combination is a well tolerated and active regimen in the treatment of older patients with MCL.
Visco:Mundipharma International Ltd: Research Funding. Off Label Use: Bendamustine has no formal indication in Italy for the treatment of mantle cell lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal