Abstract
Abstract 2357
Infection by Plasmodium Vivax (P. Vivax) is the most common cause of Sleeping Malaria. P. Vivax and other plasmodia have grown increasingly resistant to antimalarial drugs. Introduced by mosquito bite, P. vivax sporozoites enter circulation and preferentially penetrate reticulocytes by attaching to the Fya and Fyb Duffy antigen/chemokine receptor (DARC) via PvRBP-1 and PvRBP-2 proteins located at their apical poles. Once in a reticulocyte, the parasite begins to reproduce asexually, releasing of thousands of merozoites into circulation. At this point, merozoites can also enter the liver and triggering relapses months or years later.
The emergence of drug-resistant strains of p. vivax has stimulated development of new vaccines and treatments, but progress has been slowed by the dearth of reliable screening platforms. Many vaccine candidates have been developed to act upon vivax merozoites by preventing binding of PvRBP-1 and 2 to DARC, thereby arresting reproduction. However, there is a distinct lack of in vitro models to evaluate candidates that employ this mechanism. We are addressing this issue with a novel ex vivo expansion and differentiation technology for large-scale production of DARC expressing reticulocytes for in vitro P. vivax infection studies.
This technology comprises an expansion system that can produce high yields of hematopoietic precursors (CD133+/CD34+ cells) from a variety of sources (marrow, peripheral blood, and cord blood), and a differentiation system to produce a relatively pure population of enucleated erythrocytes. In this study, we have refined the polyethersulfone (PES) nanofiber-based culturing system containing growth factors and cytokines in a serum-free media, to expand hematopoietic stem and progenitor cells (HSPC) ex vivo. This expansion technology allows rapid 200-fold ex vivo proliferation within 7 days of umbilical cord blood derived CD133+/CD34+ HSPCs from a DARC+ donor.
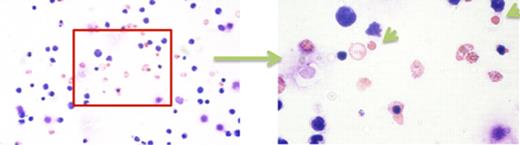
Following expansion, over 50% of these cells retained HSPC phenotype (expression of CD34+). We have subsequently demonstrated that feeder layer free three-step differentiation of nanofiber-expanded cells using cytokines results in a population containing predominately enucleated reticulocyte-like cells. At 21 days of differentiation, cells had expanded 50-fold. Around 41% of cells were enucleated reticulocytes. These cells expressed glycophorin-A, a major sialoglycoprotein present on the human erythrocyte membrane. ∼28% of cells were CD36+, and ∼70% were CD71+ indicating an erythroid lineage.
Reticulocyte-like cells and expelled nuclei during differentiation of nanofiber-expanded HSPC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal