Abstract
Abstract 2319
Thrombosomes are novel lyophilized human platelet derivatives that retain a number of key platelet structural and functional properties. Building on preliminary in vitro studies, we here sought to investigate whether Thrombosomes, suspended in platelet free plasma (PFP), would enhance and be incorporated in thrombus generated under flow conditions within a validated and well-characterised model of deep arterial injury.
PFP was obtained by centrifuging citrated whole blood from six healthy non-smoking volunteers and filtering with a 0.22μm filter. Thrombosomes were suspended in 60 mL of PFP to generate final concentrations of 20 and 200 × 106Thrombosomes /mL. Immediately prior to use, 1.2 mL of 1M CaCl2 was added to permit fibrin generation. Thrombus formation was assessed using the Badimon Chamber at low (212 s−1) and high (1690 s−1) shear rates with porcine aortic tunica media as the thrombogenic substrate. Total thrombus area and platelet-rich area were measured histomorphometrically using conventional and immunohistochemical staining respectively. Fluorescent labeled Thrombosomes were added to the extracorporeal circuit in the Badimon chamber to study the ex vivo thrombus generation in the whole blood. Electron microscopy of Thrombosomes and platelets was undertaken.
Thrombosomes contributed towards thrombus formation in whole human blood as evidenced by incorporation of fluorescent-labeled Thrombosomes into the thrombus. Immunohistochemical staining of the glycoprotein IIb/IIIa receptor confirmed incorporation of Thrombosomes into the thrombus. In the high shear chamber, mean thrombus area increased in a dose-dependent manner following the addition of Thrombosomes (704 μm2 [95% CI, 224 – 1184 μm2], 1511 μm2 [95% CI, 687 – 2336 μm2] and 2378 μm2 [95% CI, 1567 – 3189 μm2] for PFP and Thrombosomes at concentrations of 20 and 200 × 106/mL respectively [P= 0.003]). In the low shear chamber, total thrombus area for the PFP was 4962 μm2 (95% CI, 2489 – 7434 μm2). The addition of Thrombosomes at concentrations of 20 and 200 × 106/mL led to a numerical increase in mean thrombus area to 6170 μm2 (95% CI, 3944 – 8397 μm2) and 7504 μm2 (95% CI, 3864 – 11144 μm2) respectively, although this was not statistically significant (P= 0.2969).
Thrombosomes suspended in PFP and exposed to injured arterial tissue under physiologically relevant flow conditions are incorporated into thrombus and enhance thrombus formation in a dose dependent manner. These findings act as impetus to undertake clinical studies of this rehydrated, lyophilized infusible hemostatic platelet product.
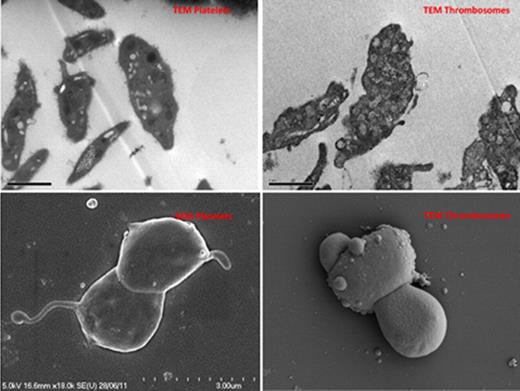
Scanning electron microscopy - Thrombosomes assumed morphology consistent with activated platelets. On transmission electron microscopy, dense granules and alpha-granules were less distinct than with normal platelets suggesting loss of cytoplasmic architecture and granule integrity.
Scanning electron microscopy - Thrombosomes assumed morphology consistent with activated platelets. On transmission electron microscopy, dense granules and alpha-granules were less distinct than with normal platelets suggesting loss of cytoplasmic architecture and granule integrity.
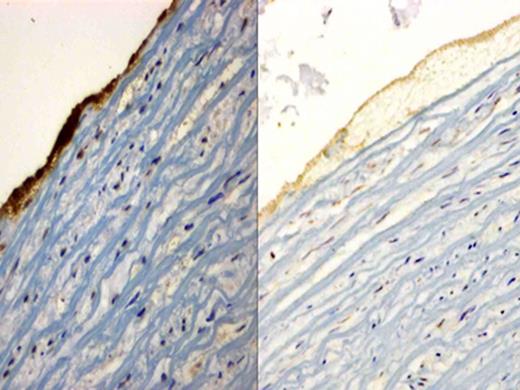
Immunohistochemical staining of glycoprotein IIb/IIIa receptor, sections were incubated with the mouse anti-human integrin alpha-2b/beta-3 monoclonal antibody and counterstained with hematoxylin. The image on the left is the representative image of thrombus caused by Thrombosomes in PFP. It shows an intense dark stain suggestive of glycoprotein IIb/IIIa receptor expression. The image on the right is PFP and only shows background staining, confirming absence of glycoprotein IIb/IIIa receptor expression.
Immunohistochemical staining of glycoprotein IIb/IIIa receptor, sections were incubated with the mouse anti-human integrin alpha-2b/beta-3 monoclonal antibody and counterstained with hematoxylin. The image on the left is the representative image of thrombus caused by Thrombosomes in PFP. It shows an intense dark stain suggestive of glycoprotein IIb/IIIa receptor expression. The image on the right is PFP and only shows background staining, confirming absence of glycoprotein IIb/IIIa receptor expression.
Total thrombus area within the high and low shear chamber. Data shown are mean ± SEM (n=6).
Total thrombus area within the high and low shear chamber. Data shown are mean ± SEM (n=6).
Fitzpatrick:Cellphire Inc.: Employment, Equity Ownership. Feuerstein:Cellphire: Consultancy. Newby:Cellphire: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal