Abstract
Abstract  2232
2232
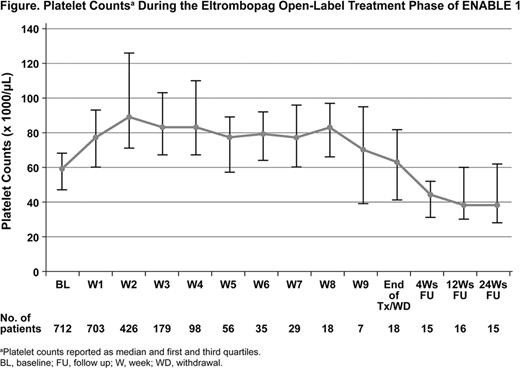
Thrombocytopenia (TCP) is a common complication of cirrhosis in patients with hepatitis C virus (HCV) infections (Louie et al 2011); the presence of TCP impairs the ability to initiate peginterferon alpha (PEG) therapy and necessitates PEG dose reduction or discontinuation, thus reducing the potential for sustained virologic response (SVR). Eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist approved for the treatment of chronic immune thrombocytopenia, increases platelet counts in patients with TCP due to HCV-related cirrhosis (McHutchison et al 2007). ENABLE 1 was a phase 3, multicenter, two-part study of eltrombopag for the treatment of HCV-associated TCP. Part 1 involved open-label, pre-antiviral treatment with eltrombopag. Patients achieving platelet counts ≥90,000/μL were randomized in Part 2 to receive eltrombopag or placebo in combination with antiviral therapy (PEG-2a plus ribavirin). Aim: To assess the safety and efficacy of eltrombopag during the open-label, pre-antiviral treatment phase (Part 1) of ENABLE 1 in patients with cirrhosis. Methods: Patients with chronic HCV and a baseline platelet count <75,000/μL were enrolled. In Part 1, all patients received open-label oral eltrombopag (25 mg daily with dose escalations every 2 weeks to a maximum dose of 100 mg) for up to 9 weeks or until platelet counts reached ≥90,000/μL. Patients who failed to achieve platelet counts ≥90,000/μL following 3 weeks of eltrombopag 100 mg daily did not enter Part 2 and attended scheduled follow-up visits. Patients achieving these counts were randomized 2:1 to eltrombopag or placebo (Part 2) at the final dose received in Part 1, in combination with antiviral therapy for up to 48 weeks. Results: A total of 716 patients were enrolled; 1 patient withdrew due to a protocol deviation, and 715 entered the open-label pre-antiviral phase. At study entry, most patients were male (62%) and Caucasian (72%); 17% were of Japanese/East Asian heritage. The median age was 52 years (range, 19–76). 488 patients (68%) had cirrhosis (FibroSURE™ score equivalent to METAVIR F4). The median duration of treatment during Part 1 was 20 days and the median of the mean daily dose was 25 mg (range, 0.8–75 mg). Median baseline platelets were 59,000/μL; these increased to 89,000/μL by week 2 and remained consistently elevated throughout open-label treatment (Figure). Following a median of 2 weeks of treatment (range, 0.1–9.6 weeks), 691 patients (97%) achieved platelet counts ≥90,000/μL. Treatment was discontinued during Part 1 for 33 patients (5%): platelets <90,000/μL (11); adverse events (AEs, 9); investigator discretion (7); patient decision (3); loss of follow-up (2); or a protocol deviation (1). During Part 2, 682 patients (95%) were randomized, 2 patients withdrew consent following randomization, and 680 patients (95%) initiated antiviral treatment. Of the patients who initiated treatment, 451 (66%) did so within 2 weeks and 627 (92%) did so within 4 weeks. The most common AEs observed during the open-label treatment phase were headache (7%), fatigue (4%), nausea (3%), and diarrhea (3%). Ninety-five patients (13%) experienced platelet counts >200,000/μL. No thromboembolic events were observed during open-label treatment. Conclusions: Eltrombopag was generally well-tolerated and resulted in sustained increase in platelet counts during the open-label, pre-antiviral treatment phase. Platelet count increases were seen as early as 2 weeks following initiation of treatment. The vast majority of patients (97%) achieved platelet count increases to ≥90,000/μL, the threshold for initiating PEG-2a plus ribavirin therapy, and most did so within 4 weeks of initiating eltrombopag treatment.
Dusheiko:GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Off Label Use: Eltrombopag, inteferon and Ribavirin; eltrombopag is a thrombopoetin receptor agonist. Its efficacy and safety in raising platelet counts in hepatitis C positive patients (most with cirrhosis) and thrombocyotopaenia was studied in this protocol. Afdhal:Merck: Consultancy, Honoraria, Research Funding; Vertex: Consultancy, Honoraria, Research Funding; Idenix: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Springbank: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Pharmasett: Consultancy, Honoraria, Research Funding; Abbott: Consultancy, Honoraria, Research Funding. Giannini:GlaxoSmithKline: Consultancy, Speakers Bureau; Hoffman-LaRoche: Consultancy, Speakers Bureau. Chen:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mostafa Kamel:GlaxoSmithKline: Employment, Equity Ownership. Brainsky:GlaxoSmithKline: Employment, Equity Ownership. Geib:GlaxoSmithKline: Employment. Vasey:GlaxoSmithKline: Employment. Patwardhan:GlaxoSmithKline: Employment, company shares. Campbell:GlaxoSmithKline: Employment, Equity Ownership. Theodore:GlaxoSmithKline: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal