Abstract
Abstract 2179
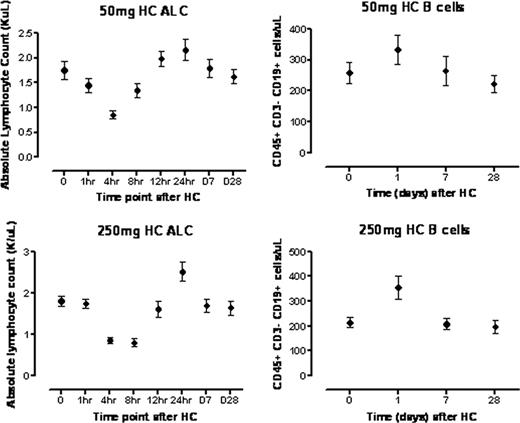
Corticosteroids have been in widespread use as anti-inflammatory and immunomodulatory agents for decades, and there is a wealth of information on their immunosuppressive effects in animals and in human cells in vitro. However, there is a paucity of data on the effects of systemically administered corticosteroids on human immune cell subsets. We used modern multiplexed techniques to query the cellular and molecular immune response, or “immunome”, in 20 healthy volunteers at baseline and after systemic (IV) hydrocortisone (HC) administered at both moderate (250 mg) and low (50 mg) doses, to provide insight into how corticosteroids exert their immunomodulatory effects at doses that are commonly used therapeutically. We performed blood draws at baseline, and then 1, 4, 8, 12, and 24 hours, and 7 and 28 days after HC infusion. We assessed whole transcriptome gene expression using HumanGene 1.0 ST microarrays on both peripheral blood mononuclear cells and whole blood; plasma levels of 69 cytokines and chemokines by luminex assay; and high dimensional comprehensive lymphocyte phenotyping by flow cytometry. We observed a global decline in circulating numbers of B and T cells which nadired 4–8 hours after the administration of both 50mg and 250mg HC. Viability and Annexin V staining for apoptosis were consistent with peripheral lympho-depletion occurring as a consequence of redistribution of lymphocytes rather than direct HC-mediated cytotoxicity. Both B and T cell numbers rebounded significantly above baseline 24 hours after HC infusion, and the extent of this increase varied among specific lymphocyte subsets, with CD45+CD3-CD19+ B cell numbers exhibiting the highest rebound over baseline values after both the 50mg (P=0.0060) and 250mg (P=0.0036) HC doses, respectively (Figure 1). In contrast, CD3-CD56+CD16+ NK cell numbers remained stable at all time points after administration of 50mg or 250mg of HC. Microarray studies revealed a steroid-responsive gene expression signature that preceded redistribution of lymphocyte subsets, with 336 genes showing significant variation as early as 1 hour after HC administration (P value cutoff <0.001), and peak changes in gene expression (n=2135, P value cutoff <0.001) occurred 4 hours after HC administration. Further analysis is ongoing to assess for hidden variables. Protein profiling demonstrated significant decreases in circulating levels of immune-related cytokines within 24 hours of HC administration, including TNF-related apoptosis-inducing ligand (TRAIL, P=0.0164), IL-1b (P=0.009), IL-2 (P=0.034), IL-4 (P=0.0104), and IFN-g (P=0.0264). In contrast, circulating levels of IL-6, IL-10, IL-15, and IL-17 remained at baseline after HC infusion. Our study is the first to systematically characterize the effects of corticosteroid administration on the human immunome in vivo, and we demonstrate that corticosteroids exert differential effects on B and T lymphocytes and natural killer cells in humans.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal