Abstract
Abstract 1910
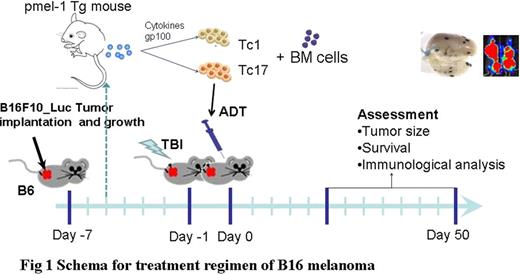
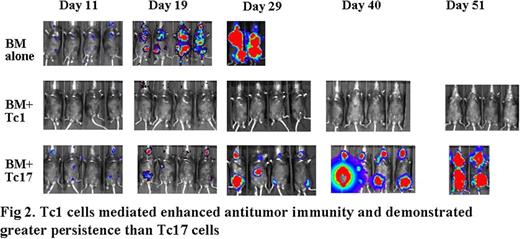
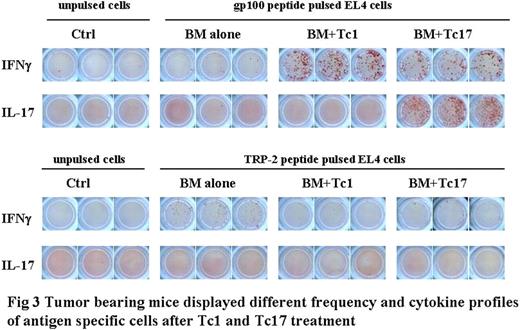
Adoptive cell transfer (ACT) of tumor-reactive T cells is one of the most promising approaches for the treatment of established melanoma. Recently, limited studies provide some evidence that Th/Tc17 cells may also have potent anti-tumor activities, but the conclusion is far from reach. Methods: Human gp10025-specific Tc1 or Tc17 cells were generated from pmel-1 transgeneic mice and used as cell source for ACT. Luciferase-transduced B16 melanoma was intravenously injected into C57BL/6 mice to establish lung-metastasis. After 7 days, tumor-bearing mice were lethally irradiated and transferred gp-10025 specific Tc1 or Tc17 cells in the combination of syngeneic bone marrow. Survival of those tumor-bearing mice was monitored daily, and tumor growth was monitored weekly using in vivo bioluminescent imaging (BLI). Donor T-cell expansion and cytokine secretion from the spleen and lung of tumor bearing mice were analyzed using flow cytometry and ELISPOT assays. To evaluate the role of IFNγ in anti-tumor immunity, we used a B16 melanoma cell line that was transduced with a plasmid encoding a dominant-negative IFNγ receptor (B16-IFNγRDN), and IFNγR knockout mice as tumor-bearers. Results: As expected, irradiation and transfer of syngeneic bone marrow had little or no effect on established melanoma. Adoptive transfer of tumor-specific Tc17 cells significantly suppressed the tumor growth, whereas Tc1 cells induced long-term regression of established melanoma. After ACT, Tc1 cells maintained their phenotype to produce IFNγ. However, Tc17 cells largely preserved their ability to produce IL-17, but a subset of them secreted IFNγ, indicating the plasticity of Tc17 cells in vivo. Mechanistically, Tc1 cells executed their anti-tumor immunity primarily through the direct effect of IFNγ on melanoma cells because Tc1 cells had essentially no effect on B16-IFNγRDN tumor. However, Tc1 cells had a similar therapeutic effect on IFNγR knockout as wild type mice, indicating that IFNγ signaling in host cells was not critical. In contrast, despite the fact that Tc17 cells also secreted IFNγ, Tc17-mediated anti-tumor immunity was independent of the effect through IFNγ. Ironically, IFNγ was inhibitory to Tc17-mediated anti-tumor activity. Conclusions: Taken together, these studies demonstrate that both Tc1 and Tc17 cells can mediate effective anti-tumor immunity, but Tc1 is superior to Tc17. These findings also demonstrated for the distinct effect mechanisms of antigen-specific Tc1 and Tc17 cells in anti-tumor response, and direct IFNγ signaling on tumor cells is a key effect to eradicate established tumors mediated by Tc1 cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal