Abstract
Carfilzomib (CFZ) is a next-generation proteasome inhibitor that selectively and irreversibly binds to its target. Phase (ph) 1 and 2 studies with CFZ have demonstrated durable single-agent activity and acceptable safety in patients (pts) with relapsed and/or refractory multiple myeloma (MM). Preliminary side-by-side comparison of efficacy results from PX-171-003-A0 and PX-171-003-A1, 2 studies performed with nearly identical entry criteria and schedules but using different doses of CFZ (20 mg/m2 vs 20/27 mg/m2), suggested a dose–response relationship. The rates of pts achieving either a partial response (PR) or better or a minimal response or better appeared to be greater in 003-A1 than in 003-A0. An analogous comparison of efficacy results from the 2 bortezomib (BTZ)-naïve dosing cohorts (20/27 mg/m2 vs 20 mg/m2) in PX-171-004 revealed similar trends. The present analysis was undertaken to rigorously evaluate the evidence of a potential dose–response relationship for CFZ by employing dose– response modelling.
A pooled multivariate dose–response model was derived using data from the following ph 2 studies of CFZ: 003-A1, 004 (BTZ-naïve), and 004 (BTZ-treated). A multivariate logistic regression analysis for the primary outcome of overall response rate (ORR) was fitted that adjusted for study, CFZ dose, and a broad range of prognostic covariates (eg, cytogenetic status and International Staging System (ISS) stage). In addition, a repeated measures model used generalized estimating equations (GEE) to analyze the association between cycle-specific response status and cycle-specific CFZ dose. Multivariate Cox regression models with the same covariates, stratified by study, were fitted for the time-to-event endpoints (duration of response [DOR], progression-free survival [PFS], and overall survival [OS]). Time-dependent Cox regression models were also fitted for these endpoints using cycle-specific cumulative dose.
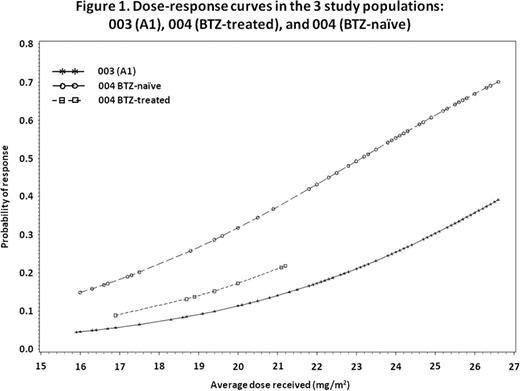
Evaluation of the effect of actual CFZ dose on the primary efficacy outcome of overall response rate (ORR) demonstrated that the odds of achieving a PR or better for a given pt treated with 27 mg/m2 were 4.08-fold higher (95% CI: 2.30–7.24, P<0.001) than for a pt receiving 20 mg/m2. When using the average dose as a continuous variable and adjusting for study effect, the odds of a response increased by 1.28-fold (95% CI: 1.17–1.40; P<0.001) for each 1 mg/m2 increase in average CFZ dose, equivalent to a 5.52-fold increase in the odds of a response if the average dose increased from 20 mg/m2 to 27 mg/m2. Figure 1 presents the dose–response curve for each of the 3 study populations derived by applying the inverse logit transformation to the estimated odds ratio.
Results were similar after adjusting for various baseline prognostic covariates across studies (eg, female gender, higher Hgb level). Multivariate Cox regression models with same prognostic factors were fitted for the secondary efficacy endpoints of DOR, PFS, and OS, and highly significant effects of CFZ dose were again observed.
A repeated measures model (using cycle-specific average dose as a dose effect variable) using GEE was applied to remove a potential bias of differential pt characteristics in those receiving higher doses of carfilzomib. In this analysis, the per-cycle odds of an overall response was estimated to increase by 1.12-fold (95% CI: 1.06, 1.18; p<0.001) for each 1 mg/m2 increase in CFZ dose. Additionally, time-dependent Cox regression models were fitted to account for the potential bias due to confounding factors. Results of this analysis confirmed the initial dose–response relationship observed.
Preliminary observations of a dose–response relationship for CFZ from ph 2 studies have been confirmed and extended using a statistically rigorous, multivariate analysis. This dose–response relationship is apparent both in terms of the proportion of responding pts across all response endpoints evaluated (ORR, DOR, PFS, and OS) and in the depth of individual responses. While a corresponding dose–toxicity analysis has not been performed to date, carfilzomib has been shown to have a similar tolerability profile when comparing 20 mg/m2 (003-A0) vs 20/27 mg/m2 (003-A1). These findings are being further assessed in exploratory clinical trials (eg, PX-171-007) evaluating higher dosing regimens.
Squifflet:International Drug Development Institute: Employment. Michiels:International Drug Development Institute: Consultancy. Siegel:Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Vij:Onyx Pharmaceuticals: Consultancy, Research Funding; Celgene: Research Funding, Speakers Bureau; Millennium: Speakers Bureau. Ro:Onyx Pharmaceuticals: Employment, Equity Ownership. Buyse:International Drug Development Institute: Employment, Equity Ownership.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal