Abstract
Abstract 1741
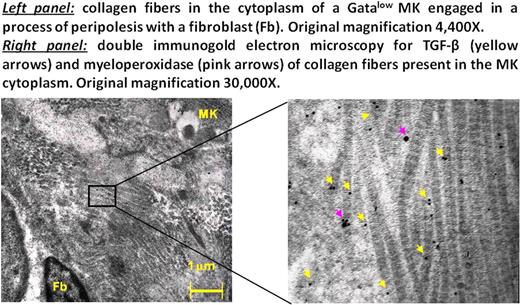
The fibrosis observed in patients with primary myelofibrosis (PMF), the most severe of the Ph-chromosome negative myeloproliferative neoplasms, and in mouse models (TPOhigh or Gata1low) of the disease is thought to be caused by fibroblasts activated by TGF-β released by megakaryocytes (MK) engaged in a pathological process of emperipolesis with the neutrophils. Gata1low mice have a life span > 2 years and develop myelofibrosis in precise sequential stages which closely recapitulate the human disease. Mice are born thrombocytopenic and anemic and remain thrombocytopenic all their life but recover from anemia at one month by developing extramedullary hematopoiesis in spleen. These mice develop fibrosis and increased angiogenesis by 6–8 months and increased hematopoietic stem/progenitor cell trafficking and extramedullary hematopoiesis in liver at 10-months (Martelli et al Blood 2005; 106:4102). Eliades et al (J Biol Chem 2011; 286:27630) recently reported that MK from Gata1low mice express high levels of lysyl oxidase, a matrix cross-linking protein responsible for collagen polymerization, identifying a an additional mechanism of fibrosis induction by Gata1low MK. By electron microscopy, we observed that 10–20% of MK in the spleen of both PMF patients and Gata1low mice contain numerous collagen fibers embedded within their cytoplasm. This observation suggested to us that lysyl oxidase-dependent collagen polymerization may occur within the cytoplasm of the MK raising the question of the mechanism that allowed collagen to enter within these cells. To clarify this issue, extensive optical and electron microscopy studies of the spleen of Gata1low mice during disease progression were performed. With disease progression, the numbers of fibroblasts in the spleen of Gata1low mice greatly increased [fibroblasts/mm2= 165±28, 245±24 and 312±60 vs 80±20 in 3-, 6- and 15-month old Gata1low mice vs 15-month-old wild type (WT) littermates, respectively p<0.05-0.01 Gata1low vs WT and 3-month vs 6- and 15-month Gata1low mice]. Furthermore Gata1low fibroblasts had an activated morphology that included long protrusions containing numerous fibronectin- and collagen-gold particles (67±10 vs 11±1 fibronectin- and 45±14 vs 13±2 collagen-gold-particles/mm2 in Gata1low and WT fibroblast protrusions, p<0.05-0.01). In numerous cases, the protrusions surrounded MK penetrating their cytoplasm in a process of peripolesis that could involve either close (see Figure) or distal cell/cell interactions. The numbers of MK engaged in fibroblast peripolesis was 23±22, 80±34 and 186±12/section in 3-, 6- and 15-month Gata1low mice vs below detection in 15-month WT littermates. Once in the cytoplasm, the fibroblast protrusions fused their membranes with those of the MK releasing fibronectin and collagen into the MK cytoplasm. MK engaged in fibroblast peripolesis presented morphological nuclear changes in which the condensed chromatin of cells was similar to that observed in para-apoptosis, a Tunel-negative process of cell death. Based on the nuclear condensation state, peripolesis-engaged MK were classified in 4 stages, the most advanced of which was characterized by the presence of collagen fibers in the cytoplasm. These collagen fibers contained great numbers of TGF-β and myeloperoxidase immunogold-particles embedded in the heavy electron dense pocket of the polymer twists (see Figure). Nude para-apoptotic MK nuclei were found surrounded by cytoplasmic MK ghosts rich in TGF-β-collagen fibers which also contained clusters of 2–6 myofibroblasts (undetected in spleen from WT mice). Fibroblast/MK peripolesis was not detectable in spleen from double Gata1lowP-selectinnull mice or in Gata1low mice treated with an inhibitor of TGF-β signaling. These mice also did not express fibrosis and neo-angiogenesis. These results indicate that fibrosis and TGF-β accumulation in the microenvironment of Gata1low mice is not a random process but occurs in “hot-spots” localized at sites of MK and fibroblasts interaction. We suggest that this previously undescribed TGF-β (to activate fibroblast)- P-selectin (to promote fibroblast/MK interaction)-dependent MK/fibroblast interaction may facilitate scaring and vessel formation not only in PMF but in other diseases as well.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal