Abstract
Abstract 1738
Since the initial description of V617F somatic mutation in patients with Philadelphia chromosome negative myeloproliferative neoplasms (MPNs), a remarkable association between alterations in the JAK2 gene and MPNs has emerged. In addition to V617F, a number of other mutations have been detected in exons 12–15 of the JAK2 gene. Furthermore, a specific JAK2 haplotype predisposes to somatic V617F mutation and MPN. However, the link between JAK mutation and MPNs is not straightforward. For example, in familial cases of MPNs, occurrence of V617F is heterogeneous and occurs as a somatic rather than germline mutation. The underlying inherited genetic abnormality in many of these cases remains unknown. Similarly, occurrence of JAK2 V617F in sporadic MPNs is also heterogeneous and is associated with variable disease characteristics. Thus, understanding of the relationship between JAK2 mutation and MPN disease phenotype remains far from complete.
We herein report a family with germline V617I mutation (Figure 1), associated with mild/moderate thrombocythaemia and thrombosis. All patients had normal haematocrit, WBC and peripheral blood (PB) morphology without splenomegaly or other abnormality on physical examination.
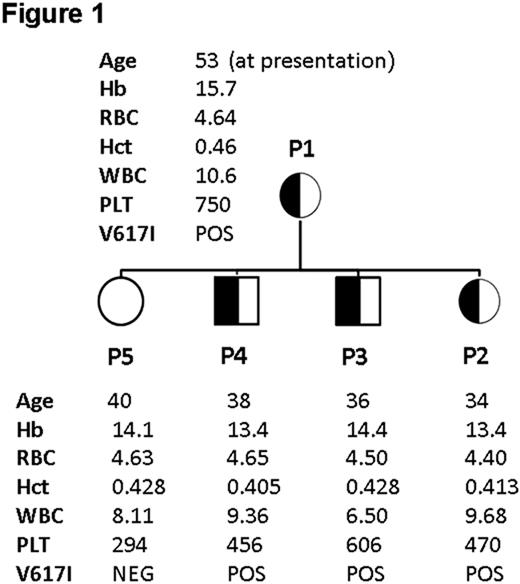
P1: Presented in 2006 at the age of 53 with a significant ischaemic cerebrovascular event and a platelet count of 750 × 109/l, with a history of longstanding thrombocythaemia (>10 years; 700–970 × 109/l). Bone marrow examination showed normal architecture with increased numbers of morphologically normal megakaryocytes and no fibrosis. She was commenced on aspirin and hydroxycarbamide resulting in good control of the platelet count. Aspirin was not tolerated due to recurrent epistaxis. Subsequent JAK2 mutation screening by pyrosequencing demonstrated an abnormal pyrogram pattern subsequently identified to be V617I by Sanger sequencing. Quantification of allelic level by a pyrosequencing assay designed to detect V617I confirmed heterozygous (≈50%) V617I in PB mononuclear cells (MNCs), CD3+ cells, CD66+ myeloid cells, buccal swab DNA and hair follicle DNA.
P2: Daughter of P1. 34 years. Asymptomatic. Persistent thrombocythemia (470–604 × 109/l). Heterozygous (≈50%) V617I in PB MNCs, CD3+ T cells, CD66+ myeloid cells, buccal swab DNA and hair follicle DNA.
P3: Son of P1. 36 years. Asymptomatic. Persistent thrombocythemia (606–648 × 109/l). Heterozygous (≈50%) V617I in PB MNCs, CD3+ T cells, CD66+ myeloid cells and buccal swab DNA.
P4: Son of P1. 38 years. Asymptomatic. Persistent thrombocythemia (456–526 × 109/l). Heterozygous (≈50%) V617I in PB MNCs, CD3+ T cells, CD66+ myeloid cells and buccal swab DNA.
P5: Daughter of P1. 40 years. Platelet count 294 × 109/l. V617I negative in all tissues.
V617I has been previously reported to occur rarely in MPN (PMID: 19074595) and to be constitutively-activating in cell line models (PMID: 18326042) and molecular dynamic simulations (PMID: 19744331).
Single cell intracellular quantitative pSTAT3 FACS analysis of PB cells from P1-4 demonstrated GCSF hyper-responsiveness of V617I positive PB CD33+ and CD34+ cells. For example, in comparison with normal controls (NC; n=6) V617I CD33+ myeloid cells (n=4) showed a 14-fold increased pSTAT3 mean fluorescent intensity relative to unstimulated cells in response to 15 minutes stimulation with 0.8 ng/ml GCSF (11% vs 156%; P<.001). BFU-E and CFU-MK in PB were not significantly different between NCs (n=4) and V617I (n=4). CFU-GM were increased in the PB of V617I patients (25 vs 46 colonies/200,000 PB MNCs; P<.05). Cytokine independent colonies were not observed. Single nucleotide polymorphism array did not reveal any additional acquired abnormalities in P1-4. None of the patients carried a JAK2 46/1 haplotype.
Patients 2–4 are currently under observation and receiving low-dose aspirin only.
Germline activating JAK2 mutation is a previously unreported cause of inherited thrombocythaemia (autosomal dominant) and should be considered in familial cases or when rare JAK2 mutations are detected. Importantly, V617F allele specific PCR, would not detect such mutations and, consequently, it is possible that similar cases may have been missed. Finally, the mild/moderate thrombocytosis (without evidence of fibrosis over 50 years) observed with human germline V617I provides fundamental insights into the role of JAK2 mutations in the pathophysiology of human MPNs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal