Abstract
Abstract 1674
Imatinib, nilotinib and dasatinib are effective therapies for chronic myeloid leukemia, as a consequence of their inhibiting the Abelson tyrosine kinase activity of the BCR-ABL oncoprotein. Their rank-order of potency in assays assessing either effects on BCR-ABL autophosphorylation or cell proliferation is dasatinib > nilotinib > imatinib. However, such quantitative in vitro differences do not directly translate into pharmacological differences due to other factors (Laneuville et al. JCO 2010;28: e169), such as pharmacokinetics (dasatinib, nilotinib and imatinib have plasma half-lives in CML patients of 3–5, 19±6 and ≈20 hours respectively) and pharmacodynamics, including cell penetration and protein binding. Here we report the ABL binding kinetics for these drugs and discuss how the data can be related to target occupancy and the pharmacodynamic properties of the drugs.
Active phosphorylated ABL (residues 118–535 of SH1 domain) was purified from insect cells and was treated with shrimp alkaline phosphatase to afford the dephosphorylated form. Drug binding kinetics were studied using the Proteros reporter displacement assay. This utilises reporter probes that bind to the target protein, with the proximity between reporter and protein resulting in the emission of an optical signal, so that compounds which bind to the same site as the reporter probe displace the probe, causing signal diminution. The rate of reporter displacement is measured over time after addition of compounds at various concentrations.
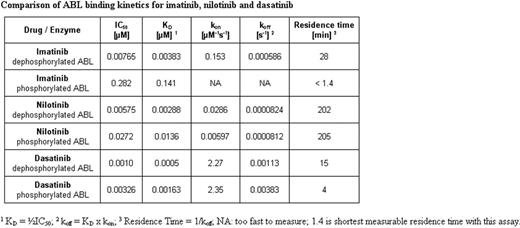
The kinetic parameters for the interaction between the drugs and either phosphorylated or dephosporylated ABL are tabulated below. The binding affinity (KD) is related to the association rate constant (kon) and the dissociation rate constant (koff) by KD = koff / kon. The kon rates (dasatinib > imatinib > nilotinib) reflect that dasatinib recognises the phosphorylated, catalytically-active resting-state conformation of ABL (DGF-in; Tokarski et al. Cancer Res 2006;66: 5790 and Vajpai et al. JBC 2008;283: 18292), whereas the other drugs bind to an inactive conformation (DFG-out) and this leads to dasatinib having the highest KD of the three drugs. However, in terms of their Koff rates, the rank order of activity is reversed, reflecting the strong binding of nilotinib to the DFG-out conformation and the thermodynamic stability of the ABL-nilotinib complex. The stability of the complexes between the drugs and ABL protein is quantifiable through the half-life of the complexes (residence times) and for nilotinib is over 3 hours.
It is generally recognised that prolonged residence-times for drugs bound to their pharmacologically-relevant protein target, can uncouple pharmacodynamic target inhibition from tissue level pharmacokinetics. Furthermore, high off-rate selectivity can minimise the side-effects of drugs caused by off-target binding, which is reduced in the case of drugs with high target residence times. Thus this kinetic analysis can provide an explanation for the high efficacy of nilotinib in CML patients, at doses that cause relatively few side-effects.
Manley:Novartis Pharma AG: Employment. Cowan-Jacob:Novartis Pharma AG: Employment. Fendrich:Novartis Pharma AG: Employment. Jahnke:Novartis Pharma AG: Employment. Fabbro:Novartis Pharma AG: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal