Abstract
Abstract 1303
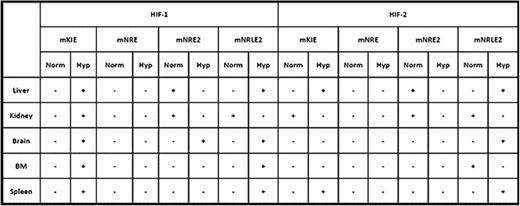
Studies of the regulation of erythropoiesis by hypoxic induction of erythropoietin (EPO) transcription led to the discovery of oligonucleotide binding motifs, hypoxia responsive elements (HREs) essential for hypoxic upregulation of EPO. HREs bind hypoxia-inducible transcription factors (HIFs). HIFs are heterodimers of 3 α subunit isotypes; HIF-1α, HIF-2α, and HIF-3α, and a common β-subunit. EPO is expressed in diverse tissues, some regulated mainly by HIF-2 and others by HIF-1. Tissue-specific regulatory elements (i.e. Kidney Inducible Element KIE, Negative Regulatory Element NRE, and Negative Regulatory Liver specific Element NRLE) of the EPO gene spanning several kilobases were reported. Our in silico analysis of the human EPO genome found two additional canonical HIF-binding elements in the KIE and NRE regions, and comparative analysis of phylogenically conserved sequences of Epo genes refined these HIF-binding elements from several kbs to a few hundred nucleotides and further delineated them as mKIE, mNRE1, mNRE2, and mNRLE2. To further define the structure and function of these elements in vivo, we exposed mice to 8% O2 hypoxia and monitored hypoxic induction of Epo mRNA levels in the liver, kidney, brain, and spleen. Spleen, kidney, and brain had a peak induction of the Epo transcript at 3 hours of hypoxia treatment, while liver showed peak induction at 6 hours. At the end of the hypoxia treatment, the mice were sacrificed and organs were harvested, and in vivo chromatin immunoprecipitation (ChIPs) was performed with antibodies against HIF-1α and HIF-2α. The results from these studies are summarized below.
“+” denotes presence and “−” absence of binding of HIF-1 and HIF-2. “Norm” - normoxia, “Hyp” - hypoxia.
To further define the role of these elements in tissue-specific Epo expression, we cloned and mutated these elements with reporter constructs, and generated transgenic mouse strains. The reporter gene was expressed in renal tubules, the red pulp area of the spleen, and liver hepatocytes. By creating a designed point mutation to disrupt the function of KIE, we suppressed the hypoxia response in the kidney, while mutations of NRE1 and NRE2 abolished reporter expression in the liver. Studies of HREs in the brain are ongoing.
In conclusion, we have refined the previously reported HREs to a few hundred bases (particularly, KIE is a 6 nucleotide bearing motif) and now demonstrate tissue-specific differential hypoxia-induced binding of HIF-1 and HIF-2 and their functional roles in EPO induction. This provides a background to examine the molecular basis of these elements in patients with aberrant EPO expression.
Elliott:Amgen, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal