Abstract
Abstract 1203
Hemophilia A is a lifelong bleeding disorder caused by a mutation(s) in the gene for coagulation factor VIII (FVIII). Desmopressin acetate (DDAVP) is an important therapy in the treatment of mild hemophilia A (FVIII >5%), although only ∼50% of patients are responsive. Baseline activity (FVIII:C) levels are expected to correlate with clinical response (higher baseline = better response) and a recent study (Castaman et al 2009) suggested that certain mutations may also influence responsiveness to DDAVP. We sought to characterize the relationship between DDAVP responsiveness and FVIII mutation in our patient population.
The Puget Sound Blood Center (PSBC) EMR database was queried with the search terms “hemophilia A” and “mild,” under an IRB approved protocol. Male subjects 12 years of age and older were included in this study if there was a genotype, a baseline FVIII:C, and a DDAVP trial result (FVIII:C peak levels collected within 1hr after DDAVP administration). Familial relationships were also recorded. Mutation analysis was performed in the PSBC Genomics Laboratory. For subjects with more than one trial, the highest peak was recorded. Complete (CR), partial (PR) and non response (NR) were classified as FVIII:C peak ≥50IU dL−1, <50IU dL−1 but increased at least 2-fold and >30IU dL−1, neither of above, respectively.
263 subjects were identified with FVIII:C >5% and 141 had genotype results. All subjects who underwent genotyping had identifiable mutations, with 139 missense mutations, 1 splice junction mutation, and 1 small deletion. There were 71 unique mutations overall (24 previously unreported in HAMSTeRS). 63 subjects fulfilled the study criteria (46 unique mutations). Results of the DDAVP trials and mutation location are in the Table below. The distribution of mutation locations was similar to previously published results. Only three of the mutations have been previously published with clinical response to DDAVP (Castaman et al 2009). There was no difference in the number of CR's to IV versus IN DDAVP. Eight genotypes (groups) were common to 2 or more subjects (n=26); 2 groups showed CR in all subjects (Asn1971Tyr n=2, Ser2192Ile n=4) and a 3rd group showed similar responses among subjects (Tyr1699Phe n=4; 3 CR and 1 PR). In three sibling pairs, 2 pairs showed complete responses (CR/CR) and one showed similar response (CR/PR). Of the 14 subjects with mutations in the C1 or C2 domain, all responded to DDAVP (11 CR, 3 PR).
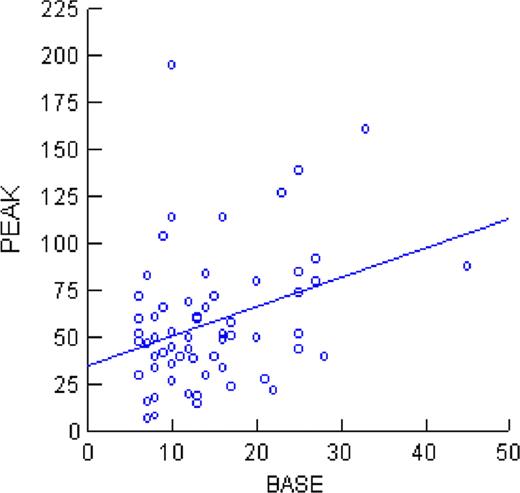
The relationship between baseline and peak FVIII:C is shown in the Figure below (r2=0.11), which reached statistical significance (P<0.05). However, this accounted for only 11% of the variability in the peak FVIII:C value with respect to baseline. Eight subjects with baseline FVIII:C ≤10% had complete responses and four subjects with baseline FVIII:C: >16% were non responders.
IV = Intravenous DDAVP, IN = Intranasal DDAVP, †mean reported in IU dL−1.
| DDAVP Response . | Total n=63 (%) . | IV n=43 (%) . | IN n=20 (%) . | Baseline FVIII:C† (range) . | Peak FVIII:C† (range) . | Increase FVIII:C† (range) . | A1 domain . | A2/a3/A3 domain . | C1/C2 domain . |
|---|---|---|---|---|---|---|---|---|---|
| Complete | 34 (54) | 24 (56) | 10 (50) | 17 (6–45) | 80 (50–195) | 64 (27–185) | 4 | 19 | 11 |
| Partial | 15 (24) | 12 (28) | 3 (15) | 12 (6–28) | 40 (24–49) | 27 (7–42) | 3 | 9 | 3 |
| None | 14 (22) | 7 (16) | 7 (35) | 12 (7–22) | 19 (7–30) | 7 (0–16) | 2 | 12 | – |
| DDAVP Response . | Total n=63 (%) . | IV n=43 (%) . | IN n=20 (%) . | Baseline FVIII:C† (range) . | Peak FVIII:C† (range) . | Increase FVIII:C† (range) . | A1 domain . | A2/a3/A3 domain . | C1/C2 domain . |
|---|---|---|---|---|---|---|---|---|---|
| Complete | 34 (54) | 24 (56) | 10 (50) | 17 (6–45) | 80 (50–195) | 64 (27–185) | 4 | 19 | 11 |
| Partial | 15 (24) | 12 (28) | 3 (15) | 12 (6–28) | 40 (24–49) | 27 (7–42) | 3 | 9 | 3 |
| None | 14 (22) | 7 (16) | 7 (35) | 12 (7–22) | 19 (7–30) | 7 (0–16) | 2 | 12 | – |
Summary/Conclusions: Genotype influences baseline FVIII and response to DDAVP; subjects with the same genotype (kindred and unrelated) have similar results. DDAVP IV and IN appear to be equally effective at eliciting a CR in patients who are responsive. Mutations in the C1 or C2 domain are consistently responsive to DDAVP, a finding also reported by Castaman et al 2009. However baseline FVIII may not be as strong a predictor of response as previously thought. While DDAVP testing is still warranted in most patients with mild hemophilia A, mutation analysis may be utilized in the future to better target therapy. Among kindred, a CR predicts a similar, favorable response and DDAVP testing may not be needed prior to clinical use for individuals within a family.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal